Julie Welburn
Mechanistic cell biology of cell division and cell architecture in health and disease

Julie Welburn is a Wellcome Senior Research Fellow in Biomedical Research. Her group is uncovering mechanisms of molecular transport that underlie cell division and cellular organisation. Most of the work involves biochemistry, structural biology, in vitro reconstitution and cell biology.
Julie did her PhD in structural biology of the regulation of cell cycle complexes at the Laboratory of Molecular Biology (LMB), University of Oxford with Prof Jane Endicott and Prof Martin Noble. She spent one year in Eva Nogales's laboratory at UC Berkeley to further study mitotic protein complexes by electron microscopy. She joined the Cheeseman lab, at the Whitehead Institute and MIT, Boston, in 2008 to pursue cell biology and biochemical studies on mitosis.
In April 2012, Julie moved to the Wellcome Centre for Cell Biology, Edinburgh to start her own research group with a CRUK Career Development Fellowship (2012-2018).
Lab members
Agata Gluszek-Kustusz, Haonan Liu, Conor Treacy, Jeraldine Weber and Huey Ying Kok
Mechanistic cell biology of cell division and cell architecture in health and disease
Research
To maintain their genomic integrity, eukaryotic cells must replicate their DNA faithfully and distribute it equally to the daughter cells. Mitotic defects lead to aneuploidy and cancer. This indicates that the mitotic mechanisms that are in place to allow faithful division have been compromised. The segregation of chromosomes is mediated by polarized and highly dynamic filaments, termed microtubules. Microtubules depend on motor proteins to assemble into a spindle and segregate chromosomes. These motors play key roles in cytoskeletal organization during cell division but also in cell migration, polarity, and axonal and cytoplasmic transport. However, the reductionist approach to studying these motors in isolation is not sufficient to understand their function in the cellular context. It remains unclear how the activities of individual motors and their interacting regulatory networks cooperate to generate physiological cellular function such as chromosome segregation. We aim to define how kinesin motors are modulated by their cargos to provide a specific output, and how the coordinated activities of kinesin motors are greater than the sum of their individual activities in vitro and in human cells.
Kinetochores and motors
CENP-E is a huge motor (730 kD), recruited to unattached kinetochores. CENP-E moves kinetochores along microtubules to facilitate chromosome alignment. How CENP-E associates with the kinetochore, how human CENP-E is activated to walk on microtubules and how CENP-E motor ensembles are coordinated to move chromosomes is currently not known. Using a non-biased approach, we recently found the C-terminal kinetochore-targeting region of CENP-E interacts with BubR1, amongst other proteins in nocodazole-arrested mitotic cell extracts. We have defined the molecular determinants that specify the interaction between BubR1 and CENP-E. The basic C-terminal helix of BubR1 is necessary but not sufficient for CENP-E interaction, while a minimal key acidic patch on the kinetochore-targeting domain of CENP-E, is also essential. We then demonstrated that BubR1 is required for the recruitment of CENP-E to kinetochores to facilitate chromosome alignment. In collaboration with the Gruneberg lab, University of Oxford, we showed this BubR1-CENP-E axis is critical to align chromosomes that have failed to congress through other pathways and recapitulates the major known function of CENP-E. Overall, our current studies define the molecular basis and the function for CENP-E recruitment to BubR1 at kinetochores during mammalian mitosis. Our future work now focused on what is the basis and function for the 2 distinct recruitment pathways of CENP-E to kinetochores and defining the activation mechanism of human CENP-E to ensure faithful mitosis.
Mitotic motors and microtubule dynamics
Our lab has made new discoveries on the mechanism of mitotic microtubule depolymerases for two families recently published: the Kinesin-8 and the Kinesin-13 motors. Our current work now addresses how MCAK and Kinesin-8 motors cooperate to control microtubule length in mitosis.
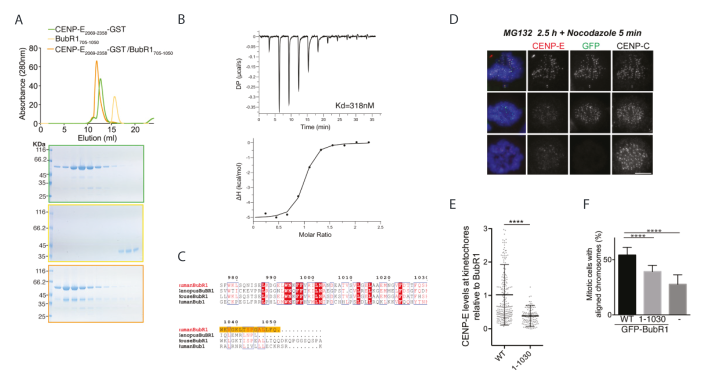
A. Top, SEC analysis and elution profile for CENP-E2069-2358-GST (green), BubR1705-1050 (yellow) and CENP-E2069-2358-GST/BubR1705-1050 (orange). Bottom, Coomassie-stained gels showing elution profiles for the corresponding protein complexes.
B. Thermodynamics of BubR1705-1050/CENP-E2091-2358-GST interaction determined by isothermal titration calorimetry. The y-axis indicates kcal/mol of injectant. The dissociation constant (Kd) between BubR1705-1050 and CENP-E2091-2358-GST was determined to be 318 ± 90 nM.
C. Sequence alignment of the C terminus of human BubR1 with mouse and Xenopus BubR1, and human Bub1. Boxed red and blue are the conserved and similar amino acids across all 4 proteins, respectively. Amino acids in red are those with conserved properties in at least 3 sequences. The sequence necessary for BubR1 binding to CENP-E2055-2608 is highlighted in orange.
D. Representative immunofluorescence images of HeLa cells treated with BubR1 siRNA and induced to express GFP-BubR1 WT and GFP-BubR11-1030, stained with CENP-E, CENP-C and Hoechst after treatment with MG132 for 2.5 hrs. Scale bar: 10 mm.
E. Scatter plot showing CENP-E intensity relative to GFP-BuBR1 at individual kinetochores plotted as grey circles, with mean and standard deviation represented by black lines.
F. Graph showing percentage of cells with at least 1 misaligned chromosome for BubR1-depleted cells induced to express GFP-BubR1, BubR11-1030 or without induction. Error bars represent standard deviation. **** indicating P < 0.0001.
Selected publications
Legal T., Hayward D., Gluszek-Kustusz A., Blackburn EA., Spanos C., Rappsilber J., Gruneberg U., Welburn JPI. The C-terminal helix of BubR1 is essential for CENP-E-dependent chromosome alignment. JCS (2020). 133:16. PMID: 32665320.
McHugh T, Zou J, Volkov V, Bertin A, Rappsilber J, Dogterom M, Welburn JPI. The depolymerase activity of MCAK shows graded response to Aurora B phosphorylation through allosteric regulation. JCS. (2019). PMID: 30578316
McHugh T., Gluszek-Kustusz A., Welburn JPI. Kinesin-8 motor Kif18b tethering to microtubule ends is a requirement for spindle positioning. JCB. (2018). 217:7. 2403-2416. PMID: 29661912

