Ken Sawin
Cell polarity and cytoskeletal organisation

Ken Sawin is a Wellcome Investigator and Professor of Cell Biology at the University of Edinburgh. His group studies multiple aspects of cell organisation, from microtubule nucleation mechanisms to cell polarity regulation, primarily using fission yeast S. pombe as a model organism. The group uses a range of experimental methods, including classical and molecular genetics, imaging, biochemistry, proteomics and structural biology.
After studying Physics and Philosophy as an undergraduate at Yale, Ken did his PhD in Cell Biology with Tim Mitchison at the University of California, San Francisco, where he developed a system for mitotic spindle assembly in vitro and discovered the major spindle kinesin Eg5. He then moved to London as a postdoctoral fellow with Paul Nurse at the Imperial Cancer Research Fund (now Cancer Research UK), where he began his work with fission yeast.
Ken joined the Wellcome Centre in 1999 as a Wellcome Senior Research Fellow, and in 2010 he joined the Academic Staff at the University of Edinburgh. He has been Professor of Cell Biology since 2012.
Lab members
Gaurav Barve, Beste Bayrak, Tanya Dudnakova, Ankita Gupta, Adam Kovac, James Le Cornu, Domenico Modaffari, Monique Scott and Ye Dee Tay
Cell polarity and cytoskeletal organisation
We are interested in two general areas related to cellular organisation: 1) regulation of cell polarity, under both normal and stress conditions, and 2) the molecular mechanisms underlying microtubule nucleation. In both areas we use fission yeast Schizosaccharomyces pombe as a model single-celled eukaryote. We combine classical and molecular genetic analysis with live-cell fluorescence microscopy, biochemistry, proteomics/phosphoproteomics, and structural biology methods.
Cell polarity in fission yeast is regulated by multiple internal cues that cooperate and compete with each other. The Rho-family GTPase Cdc42 and its associated regulators and effectors control the actin cytoskeleton and exocytosis. Microtubules provide an additional level of control, through the microtubule-associated protein Tea1 and its interactors. We have shown how the Tea1/microtubule system coordinates polarity regulation by a conventional Cdc42 guanine-nucleotide exchange factor, Scd1, with regulation by an unconventional exchange factor, Gef1. Our work has also led to the discovery of new cell-polarity regulators outside of the Cdc42- and Tea1/microtubule-based systems, and a new understanding of how the conserved NDR kinase Orb6 regulates cell polarity. A major current focus is on how the stress-activated kinase Sty1 (homolog of human p38 MAP kinase) regulates cell polarity; we are addressing this through large-scale phosphoproteomics and genetics approaches.
Microtubule nucleation depends on the γ-tubulin complex, a large multi-protein complex enriched at microtubule organising centres such as the centrosome. Many aspects of γ-tubulin complex regulation remain a mystery. We discovered the fission yeast proteins Mto1 and Mto2, which form an oligomeric "Mto1/2 complex". The Mto1/2 complex targets the γ-tubulin complex to different sites in the cell and also activates γ-tubulin complex. Mutations in the human homolog of Mto1 lead to the brain disease microcephaly. Our current work involves understanding the mechanism of γ-tubulin complexl activation by the Mto1/2 complex, using yeast genetics, microscopy, and biochemistry approaches. In recent work we have reconstituted multi-protein complex-dependent microtubule nucleation in vitro using purified proteins, and we have characterised elements of functional nucleation complexes through cross-linking mass spectrometry as well as X-ray crystallography. We have also developed new methods to interrogate protein-protein interactions in complex “solid-phase” subcellular structures in vivo, and we have used these to investigate how Mto1/2 complex is localised to nuclear pores.
In all of our work we adopt and develop new tools and techniques as necessary to address the biological questions of interest.
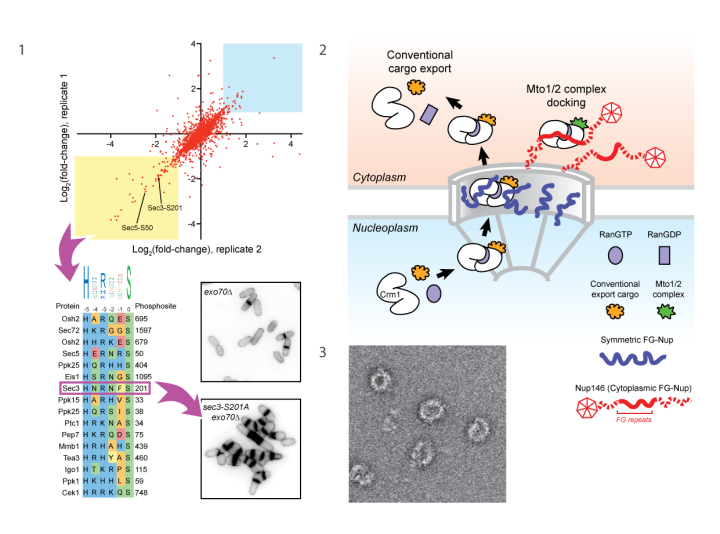
Figure 1. Global phosphoproteomics after inhibition of NDR kinase Orb6 in vivo. Many phosphorylation sites with decreased phosphorylation after Orb6 inhibition match the NDR consensus. Phosphosite mutation of Sec3 (component of exocyst complex) impairs exocytosis and cell separation after cytokinesis.
Figure 2. Model for docking Mto1/2 microtubule nucleation complex at the nuclear pore. Mto1 mimics a nuclear export cargo but uses this for docking at the pore, not for nuclear export.
Figure 3. Negative-stain electron microscopy of reconstituted fission yeast γ-tubulin ring complex.
Selected publications
Ashraf, S., Tay, Y.D., Kelly, D.A., and Sawin, K.E. (2021). Microtubule-independent movement of the fission yeast nucleus. J Cell Sci. Feb 23:jcs.253021 PMID: 33602740
Leong, S.L., Lynch, E.M., Zou, J., Tay, Y.D., Borek, W.E., Tuijtel, M.W., Rappsilber, J., and Sawin, K.E. (2019). Reconstitution of Microtubule Nucleation In Vitro Reveals Novel Roles for Mzt1. Curr Biol 29, 2199-2207 e2110. PMID: 31287970
Tay, Y.D., Leda, M., Spanos, C., Rappsilber, J., Goryachev, A.B., and Sawin, K.E. (2019). Fission Yeast NDR/LATS Kinase Orb6 Regulates Exocytosis via Phosphorylation of the Exocyst Complex. Cell Rep 26, 1654-1667 e1657. PMID: 30726745

