Owen Davies
Structural biology of meiosis

Owen Davies is a Wellcome Senior Research Fellow at the Wellcome Centre for Cell Biology. After graduating from the University of St. Andrews with a BSc (Hons) in Medical Science in 2003, he studied for a PhD at the University of Cambridge with Prof. Sir Tom Blundell and Prof. Luca Pellegrini. Upon completion in 2007, he was awarded a Royal Commission for the Exhibition of 1851 Research Fellowship for postdoctoral study at the University of Edinburgh with Prof. Sally Lowell. He then returned to the University of Cambridge in 2010, where he established his career research interest in the meiotic synaptonemal complex in Prof. Luca Pellegrini’s laboratory.
Owen was awarded a Wellcome Sir Henry Dale Fellowship in 2014 to establish his independent laboratory to study the synaptonemal complex at the University of Newcastle. In 2021, he moved to the Wellcome Centre for Cell Biology at the University of Edinburgh as a Wellcome Senior Research Fellow (2021-2026) to study the structural biology of synaptonemal complex, recombination and chromosomal dynamics in meiosis.
Lab members
Eleanor Casey, Beimi Cui, Simona Debilio, Noor Fatima Khalid, Gurusaran Manickam and Miguel Pachon
Structural biology of meiosis
How is the chromosome number halved during meiosis to create haploid spermatozoa and oocytes that form healthy diploid zygotes upon fertilisation?
Meiosis involves a unique and elegant chromosome choreography in which chromosomes search throughout the cell to find their homologous partners, with which they undergo close synapsis and exchange genetic material by crossing over, before segregating upon cell division. This is achieved by the combined actions of several elaborate molecular machines. Firstly, double-strand breaks are induced across the genome, triggering recombination searches in surrounding chromosomes which lead to the establishment of recombination intermediates that physically connect matching sequences of homologous chromosome pairs. This audacious task is facilitated by rapid chromosomal movements, which are achieved by the tethering of chromosome telomere ends to the nuclear envelope by the meiotic telomere complex, which connects chromosome ends to the LINC complex that crosses the nuclear membrane and binds to cytoplasmic microtubules. This allows microtubule forces to be transmitted to chromosomes such that microtubules act as the puppeteers of the meiotic chromosomal dance. Once established, the discrete physical connections of recombination are converted into continuous synapsis between homologous chromosomes by assembly of the synaptonemal complex, a supramolecular protein structure that ‘zips’ together homologous chromosome pairs along their entire length. The assembled synaptonemal complex then facilitates the resolution of recombination intermediates, with the formation of crossovers in which diversity is enhanced by the exchange of genetic material between homologous chromosome partners that subsequently segregate into daughter cells.
Our research aims to uncover the structural basis of how the synaptonemal complex, recombination machinery, meiotic telomere complex and meiotic LINC complex perform their critical functions in meiosis, and crucially how they operate together as an integrated molecular machine of meiotic chromosome synapsis and genetic exchange.
Our main research questions are:
- What is the structure, function and assembly mechanism of the synaptonemal complex?
- How is meiotic recombination regulated within the synaptonemal complex?
- How are meiotic chromosome telomere-ends anchored to the nuclear envelope?
- How are cytoskeletal forces transmitted to chromosomes by the meiotic LINC complex?
We adopt a structural biology approach in which we integrate solution biophysics (including multi-angle light scattering and small-angle X-ray scattering), high-resolution structure determination by X-ray crystallography and Cryo-EM, with EM-based imaging of macromolecular assemblies formed by recombinant proteins and within heterologous cellular systems. We translate our structural findings to a functional understanding of meiosis through the structure-directed design of separation-of-function mutations that are tested in vivo, in mouse and lower organism systems, by our collaborators.
Ultimately, we aim to achieve a complete molecular understanding of how the integrated machineries of the synaptonemal complex, recombination, telomere complex and LINC complex perform the elaborate chromosome choreography of meiosis, and crucially how their dysfunction leads to human infertility, miscarriage and aneuploidies such as Down’s syndrome.
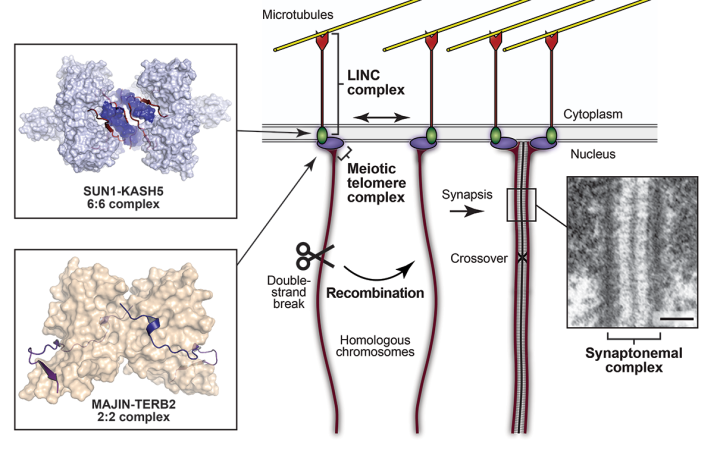
Schematic of recombination and chromosome synapsis in meiosis, highlighting the meiotic LINC complex formed of SUN1-KASH5 (Gurusaran and Davies, 2021), the meiotic telomere complex containing MAJIN-TERB2 (Dunce et al, 2018), and the synaptonemal complex (electron micrograph reproduced from Kouznetsova et al, 2011; doi:10.1371/ journal.pone.0028255).
Selected publications
Dunce, J.M., Salmon, L.J., and Davies, O.R. (2021). Structural basis of meiotic chromosome synaptic elongation through hierarchical fibrous assembly of SYCE2-TEX12. Nat Struct Mol Biol 28, 681-693.
Gurusaran, M., and Davies, O.R. (2021). A molecular mechanism for LINC complex branching by structurally diverse SUN-KASH 6:6 assemblies. eLife 10.
Sanchez-Saez, F., Gomez, H.L., Dunne, O.M., Gallego-Paramo, C., Felipe-Medina, N., Sanchez-Martin, M., Llano, E., Pendas, A.M., and Davies, O.R. (2020). Meiotic chromosome synapsis depends on multivalent SYCE1-SIX6OS1 interactions that are disrupted in cases of human infertility. Science advances 6.

