Dr Ananda Mirchandani
My research interest is understanding how critical illness, in particular critical illness-induced bone marrow hypoxia, alters monopoeisis and whether this has long-term consequences for immunity in ICU survivors.
Dr Ananda Mirchandani
Senior Clinical Research Fellow

Contact details
- Work: 0131 518 175
- Email: Ananda.Mirchandani@ed.ac.uk
Background
In adults blood monocytes are predominantly formed within the bone marrow (BM) and are recruited to peripheral organs during homeostasis to replenish a niche or to inflamed tissues when required. Within a tissue, they can become tissue-resident cells, acquiring their phenotype through cues in their environment. In recent years, however, the ontogeny of BM monocytes has been revisited, demonstrating that their ontogeny varies, during emergency monopoiesis, depending on the inflammatory cause driving this. An unanswered question in this field is whether the ontogeny of the monocyte may dictate its function within the tissue it is recruited to, irrespective of local cues.
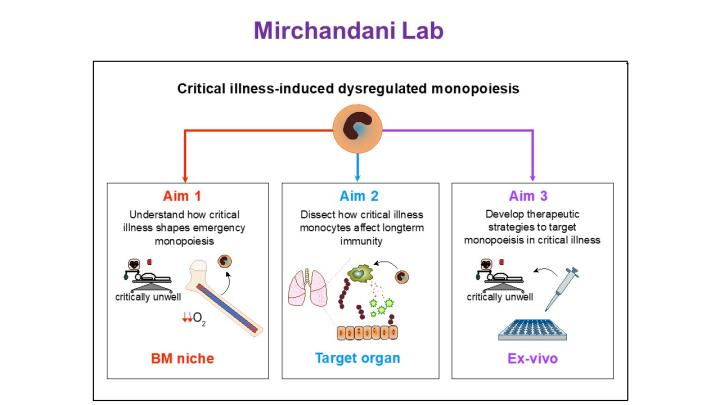
Patients who survive an Intensive Care Unit (ICU) admission remain at a higher risk of death for many years following their admission, and infection is a common cause for readmission to hospital. I have already shown that systemic hypoxia alters monopoiesis, however, the precise mechanisms driving this phenomenon and its long-term consequences remain unexplored.
Research Overview
My lab aims to dissect the molecular mechanisms that drive ICU-associated altered monopoiesis and determine if these have long-term consequences for the host’s immune function.
Using human samples and carefully curated rodent models, alongside cutting-edge methods within the IRR (including multi-colour and spectral flow cytometry, scRNASeq, spatial analysis, in-vivo multiphoton imaging and immunofluorescence) I aim to answer these questions, with a view to identify therapeutic targets to treat this unmet clinical need.
As part of this process, I hope to work alongside, and train enthusiastic, driven and ambitious scientists to become the next generation of leading PIs.
Biographical Profile
Having completed my medical degree in the University of Aberdeen with Honours, I started my clinical training in Glasgow. In 2008 I started my PhD in the Institute of Inflammation and Immunity, funded by an MRC Clinical Research Training Fellowship under the supervision of Prof Eddy Liew and Neil Thomson. I investigated the role of interleukin (IL)-33 in allergic lung disease and made some novel discoveries on the role of innate lymphoid cells type 2 in shaping adaptive immune responses. After a further period of clinical training in the West of Scotland, as a Respiratory Medicine Trainee, I was awarded a Wellcome Trust Post-doctoral Training Fellowship for Clinicians (2016-2021). I investigated the role of systemic hypoxia and ARDS in shaping innate immune responses. A key finding from this research is that systemic hypoxia shapes haematopoietic responses, shifting monocytes towards a pro-inflammatory phenotype, with consequences for inflammation resolution.
Recent publications
- Hypoxia shapes the immune landscape in lung injury promoting inflammation persistence. Mirchandani AS, Jenkins SJ, Bain CC, et al. Nat Immunol 23, 927–939 (2022)
- IL4Ra signalling abrogates hypoxic neutrophil survival and limits acute lung injury responses in vivo. Harris AJ* and Mirchandani AS*, Lynch RW, Murphy F, Delaney L, Small D, Coelho P, Sadiku P, Griffith D, Dickinson R, Clark E, Willson J, Watts E, Mazzone M, Carmeliet P, Ghesquiere B, O’Kane C, Jenkins SJ, Whyte MKB and Walmsley SR. Am J Respir Crit Care Med. 2019 Jul 15;200(2):235-246 *authors contributed equally
- Type 2 Innate lymphoid cells drive CD4+ Th2 cell responses. Mirchandani A, Besnard A, Yip E, Scott C, Bain C, Cerovic V, Salmond R, Liew FY J Immunol. 2014, 1;192(5):2442-81;192(5):2442-8 Recommended by Faculty 1000 as 2 star paper
- Interleukin-33 induces innate lymphoid cell-mediated airway inflammation by activating mammalian target of rapamycin. Salmond R*, Mirchandani A*, Bain C, Besnard A, Liew F, J Allergy Clin Immunology. 2012 Nov; 130(5): 1159-66 *authors contributed equally
Funding
Academy of Physicians of Great Britain and Ireland Young Investigator Award

