Epigenetics regulation of endothelial cells in vascular disease
Post ischemic angiogenesis requires rapid establishment of repair machinery to enhance pro-angiogenic gene expression, as a primary requisite for vascularisation and tissue regeneration. A small number of studies have identified long non-coding RNAs (lncRNAs) that have importance for endothelial cell biology in general, but their function and consequences in angiogenesis are poorly defined. One of the major functions of lncRNAs is to regulate the target-coding gene expression by association with chromatin.
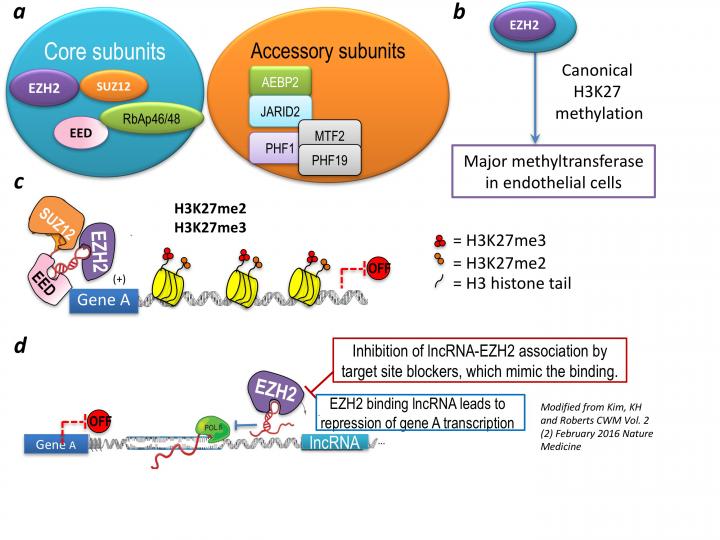
Reduced blood flow to the limbs, due to damage to the vessels, is a major cause of peripheral arterial disease (PAD) that currently lacks effective treatments. This condition may also be reducing blood flow to the heart and brain in healthy and in diabetic patients. The main priority is to re-grow the injured region and re-establish blood flow – a process known as angiogenesis. This process involves activation of the cells covering the vessel wall, also known as endothelial cells. Within, there are regions of DNA, known as genes, that encode functional RNA, and retain instructions for making the protein products. Amongst RNA the long non-coding RNAs (lncRNAs) are non-protein coding regions. Numerous lncRNAs have been discovered but without assigned function, let alone their actions in blocking angiogenesis. lncRNAs commonly regulate the human genome without changing DNA information, better known as epigenetics. In epigenetics, certain proteins modify DNA with chemical molecules adding additional information about how, when, and where to use the regions of DNA – these proteins are termed regulatory proteins because they regulate how the genes are used. One such important protein is called Polycomb receptor complex 2 (PRC2). It has been demonstrated that majority of lncRNAs interact with PRC2 to block production of proteins necessary for repair, and can impair restarting of the blood flow. However, it is not known how lncRNAs in endothelial cells influence PRC2 activity after injury or during angiogenesis. In my work I will investigate how lncRNAs engage with the regulatory proteins to then regulate DNA regions after injury to the vessels.
Research Methods and Objectives
Objectives
Several lncRNAs interact with repressive EZH2 component of PRC2 complex, that is a major histone H3K27 methyltransferase in the endothelial cells, which negatively regulates transcription. Blocking the EZH2 binding or its direct association with lncRNA could potentially modify pro-angiogenic gene expression and functional consequences thereof.
Methods
For this work, we use genomics, bioinformatics, and pharmacological approaches to study above interactions and manipulate them with a focus on assessing their mechanistic influence and impact on promoting post-ischemic reparative angiogenesis.
Principal Investigator, Co-Investigators, Other researchers
Dr Tijana Mitić, PI
Prof Andrew Baker, co-PI
Mrs Pamella Holland, technician

