Genome engineering facilities including primordial germ cell (PGC) technology
Genome engineering technologies can create precise changes to the genome, creating novel avian models for biomedical and agricultural research.
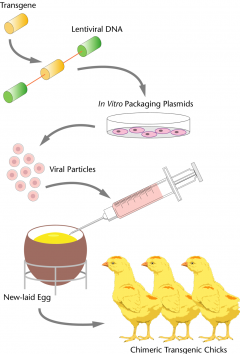
First steps in the production of transgenic chickens
Compared with mammals, producing genetically modified birds has been more challenging due to the complex structure of the avian zygote (fertilized egg)1 and the different organisation of the avian embryo2.
The first genetically modified chicken was generated in 1987, over 10 years after the creation of the first genetically modified mouse, by the insertion of retroviral foreign DNA delivered by avian leukosis virus. The retroviral vector was injected into the yolk sac near the developing blastoderm and the vector was successfully integrated into the germline3. After that, various viral vectors were used to generate genetically modified chickens4. But these viral vectors had drawbacks, such as difficulties involved in the production of replication-deficient viral vectors and risks of recombination with wild-type viruses4. These disadvantages were avoided by using plasmid-DNA microinjection into the germinal disk of the chicken zygote5 by researchers at the Roslin Institute. This led to the generation of transgenic chickens expressing neomycin resistance and a reporter gene lacZ5. However, relatively few of the chicks generated survived to sexual maturity (5.5%), and the transmission rate of the gene edit to offspring for a single cockerel was only 3.4%5.
Advancing genetic modification in chickens
Researchers at the Roslin Institute, McGrew and colleagues6 improved germline transmission of integrated transgenes by using transduction with lentiviral vectors. Lentiviral vectors were injected into the sub-germinal cavity of newly laid eggs. In this case, ten of the founder males transmitted 4-45% of the foreign DNA to their offspring6. Lentiviral vectors provided a more efficient method to generate genetically modified chickens with improved germline transmission. This technique and similar methods were used to create a number of the transgenic reporter lines that are available through the NARF today. Nevertheless, this process continued to have drawbacks, for example, the size of the transgene was still limited, modification of genes in the genome was not possible, and the introduced DNA was inefficient at modifying the chicks’ own genetic material6.
Current gene editing facilities at NARF

Primordial germ cells (PGCs) are avian stem cells that give rise to sperm or eggs. Building upon research by van de Lavoir and colleagues7, Roslin scientists with strategic support from BBSRC, increased the efficiency and duration with which PGCs taken from developing embryos can be grown in culture8. These cultured PGCs can be modified in the laboratory by insertion of a foreign gene (transgenesis) or the introduction of specific changes in the genome (editing)9. The efficiency of genome editing has been greatly increased by these advances, as well as new methods of gene editing including the CRISPR/Cas9 system which allows single nucleotide changes to be introduced at target sites in the chicken genome. When edited PGCs are microinjected into the blood supply of a developing sterile surrogate host embryos10 the genetically altered PGCs migrate to the testes or ovaries, where they give rise to sperm or eggs once the animals mature. Using sterile surrogate hosts avoids the issue of the introduced DNA competing with the chicks’ own genetic material. Mating these animals then allows chicks that carry the introduced genetic changes to be produced at high frequency.
Publications
- Mozdziak, P. E. & Petitte, J. N. Status of transgenic chicken models for developmental biology. Dev. Dyn. 229, 414–421 (2004).
- Stern, C. D. The marginal zone and its contribution to the hypoblast and primitive streak of the chick embryo. Development 109, (1990).
- Salter, D. W., Smith, E. J., Hughes, S. H., Wright, S. E. & Crittenden, L. B. Transgenic chickens: Insertion of retroviral genes into the chicken germ line. Virology 157, 236–240 (1987).
- Sid, H. & Schusser, B. Applications of gene editing in chickens: A new era is on the Horizon. Frontiers in Genetics 9, 456 (2018).
- Love, J., Gribbin, C., Mather, C. & Sang, H. Transgenic birds by DNA microinjection. Bio/Technology 12, 60–63 (1994).
- McGrew, M. J. et al. Efficient production of germline transgenic chickens using lentiviral vectors. EMBO Rep. 5, 728–733 (2004).
- Van De Lavoir, M. C. et al. Germline transmission of genetically modified primordial germ cells. Nature 441, 766–769 (2006).
- Whyte, J. et al. FGF, insulin, and SMAD signaling cooperate for avian primordial germ cell self-renewal. Stem Cell Reports 5, 1171–1182 (2015).
- Macdonald, J. et al. Efficient genetic modification and germ-line transmission of primordial germ cells using piggyBac and Tol2 transposons. Proc. Natl. Acad. Sci. U. S. A. 109, 8803 (2012).
- Taylor, L. et al. Efficient TALEN-mediated gene targeting of chicken primordial germ cells. Development 144, 928–934 (2017).


