Parkinson’s is a progressive neurodegenerative condition that affects millions of people world-wide.
Our lab is focussed in two main areas (i) understanding how the protein, alpha-synuclein, causes degeneration of neurons in Parkinson’s, and (ii) producing a cell-based therapy for Parkinson’s. Central to this aim is generation of midbrain dopaminergic neurons from human pluripotent stem cells. We use patient-derived material to generate induced pluripotent stem cells (iPSCs) with genetic mutations know to cause Parkinson’s.
Aims and areas of interest

The primary aim of the lab is to understand how disease proteins, such as α-synuclein, cause neurodegeneration, and use this understanding to generate novel therapeutics.
Background
Considerable evidence suggests that the aggregation of naturally unfolded proteins is the driving force underlying most neurological diseases. For Parkinson’s disease, the major player is the 140 amino acid protein, α-synuclein. Under physiological conditions this protein exists as a disordered random coil. However, in the presence of lipid membranes the N-terminus adopts an α-helical structure. This conformational change is important for its normal function in the release of pre-synaptic vesicles. In Parkinson’s disease α-synuclein forms many different pathological species, including long fibrillar structures that are rich in β-sheets, and contribute to Lewy body formation. Pre-fibrillar oligomeric structures have been proposed to be the most toxic, and also to be the form of α-synuclein that can spread from neuron-to-neuron in a prion-like manner. Understanding the mechanics of oligomer formation and the transfer between neurons will be a significant step towards the establishment of drug discovery platforms.
Approach and progress
We have established human pluripotent stem cell models of α-synuclein pathogenicity. We used reprogramming technology to generate a collection of induced pluripotent stem (iPS) cell lines from two members of an Iowa kindred with autosomal dominant Parkinson’s with dementia. One set of iPS cell lines carries a triplication of the gene encoding α-synuclein, and the second set of lines from a 1st-degree relative that is wild-type for this region.
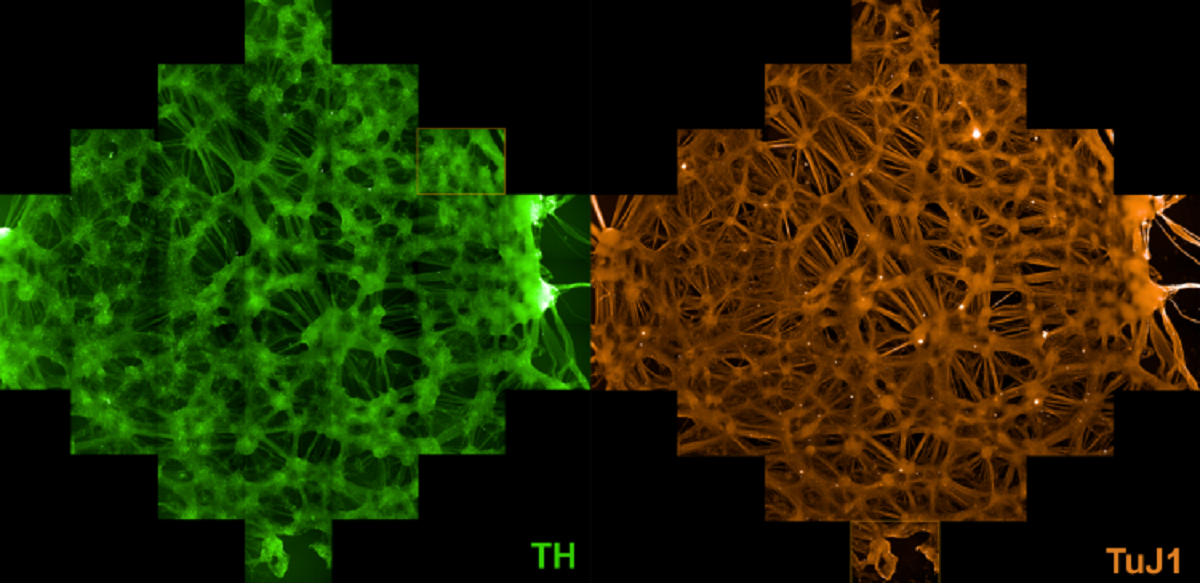
We can use the cell lines we developed to generate neurons expressing twice the amount of α-synuclein protein as wild-type neurons. To complement the iPS cell models we have also constructed transgenic human ES cell models that over-express varying levels of α-synuclein. We have an allelic series of hES and iPS cell lines to investigate phenotype severity as it relates to the degree of over-expression of α-synuclein. This collection of cell culture models will provide a platform to screen compound libraries to identify drugs that reduce or prevent the pathological behaviour of α-synuclein.

