Duncan Sproul: Epigenetics in Human Disease
Research Programme
Summary
The major aim of our programme is to understand the role that epigenetics plays in human disease.
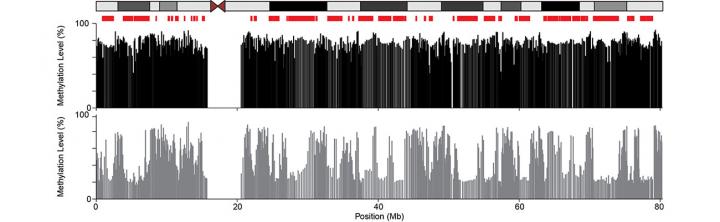
Our genomes are packaged up with the nucleus and decorated with an array of covalent modifications termed epigenetic marks. The correct patterning of epigenetic marks is thought to confer cell identity and is established during development. However, these patterns show variation between people and are disrupted during aging and disease. We do not currently understand the implications of these epigenetic alterations, and this is hindered by our lack of knowledge about how they occur. We take an interdisciplinary approach to this problem and combine computational, modelling and experimental approaches. Our lab aims to delineate the molecular mechanisms that underpin changes in the patterning of the repressive epigenetic mark DNA methylation that are observed in cancer and human populations. We focus on two broad questions:
What drives DNA methylation alterations in cancer?
Alterations to DNA methylation in cancer were first reported in 1983. However, the role of these alterations in carcinogenesis and tumour progression remains controversial. We combine the analysis of genome-wide sequencing data from patient samples with careful experiments in cellular models to dissect out the mechanisms that underpin them. Our work leverages genetic approaches through genome editing, analysing the effects of sequence variation and making comparisons to genetic conditions caused by the mutation of epigenetic regulators. We primarily focus on breast and colorectal cancer but hope that our work reveals general insights that can be applied across all cancer types.
Which mechanisms underpin DNA methylation variation in human populations?
The development of rapid epigenome profiling technologies has revealed that DNA methylation patterns vary between individuals and as we age. While these alterations are excellent epidemiological biomarkers, their implications for human health remain unclear because we do not understand the mechanisms generating them. We seek to understand why DNA methylation patterns vary across individuals and with age. Working with collaborators, we analyse and model epigenetic patterns in population cohorts and rare disease cases.


