What is regenerative medicine?
Regenerative medicine is the process of replacing or regenerating damaged or diseased human cells, tissues or organs to restore normal function. Stem cell research plays a central role in regenerative medicine, which includes the development and use of stem cell treatments.
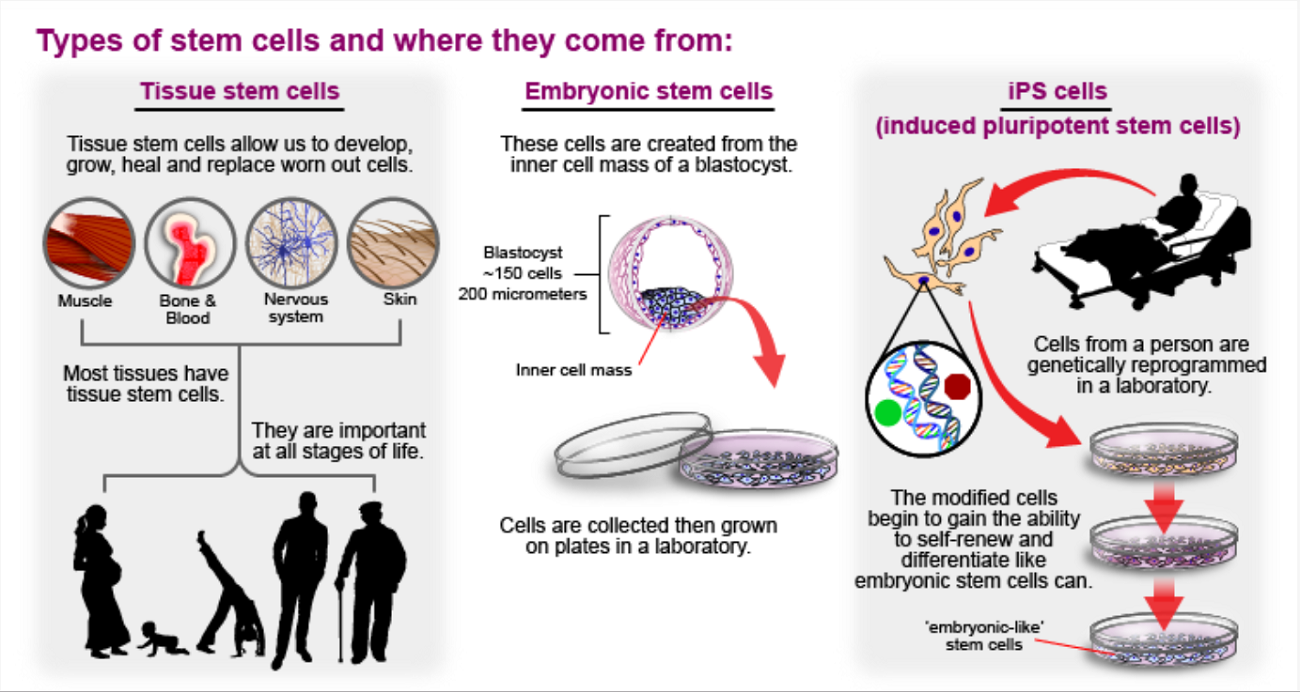
What are stem cells?
Stem cells are the body’s natural reservoir. We need to make new cells all the time to keep our body functioning. Stem cells are used to replenish stocks of specialised cells that have been damaged or used up.
Stem cells are defined by two key characteristics:
- the ability to continuously divide to generate exact copies of themselves in a process called self-renewal;
- the ability to change into specialised cells in a process called differentiation.
All multicellular animals and plants rely on stem cells to grow from a single cell into an adult. Stem cells allow our bodies to build new tissue, such as new muscle when we exercise. Stem cells also continually replace the many specialised cells in our body if they are worn out or damaged. This allows us to heal broken bones and replace skin damaged by cuts and burns.
All of this makes stem cells extremely important in the process of development, cell renewal and healing. Researchers hope that understanding how stem cells work will help to develop new treatments for many different diseases.
Visit EuroGCT to find out more about stem cells
Glossary of scientific terms used in stem cell research
Stem cell treatments
Stem cell based therapies are already used to treat patients, including bone marrow transplants for leukaemia, skin grafts for severe burns, and more recently corneal grafts for loss of sight due to ocular burns or infection. More stem cell therapies wil be developed, however some scientists and clinicians expect it will take at least 20 years before stem cell treatments become widely available.
Developing new medical treatments is a long process with many steps to ensure safety and effectiveness. New ideas for treatments must first be developed and rigorously tested in laboratories before being tested on people in clinical trials. The development of a new treatment can take around 15 to 20 years, however most ideas don’t ever become approved treatments.
Unapproved stem cell treatments are sometimes offered by unregulated companies and clinics. Unapproved procedures often lack scientific evidence showing they work and may even be dangerous. It is important that patients discuss medical decisions with their general practitioner (GP) before seeking out medical treatments.
If you are a patient seeking further information about stem cell treatments and clinical trials, please visit our Information for Patients web page. Unfortunately we are unable to give advice on, or recommend a specific treatment, stem cell therapy or clinical trial.

