Proteins have a role in every process in our body, which means they're involved in many different health conditions. Glycans are sugar structures attached to the surface of most proteins and can greatly impact protein structure and function. Glycosylation is the process by which these glycans attach to proteins. This attachment process is aided by another type of protein - called enzymes. While proteins themselves are commonly studied, sugars are often neglected. Like studying a bird without all its feathers, studying proteins without considering their glycosylation would make us miss the complete picture.
Volunteer data, from the Orkney Complex Disease Study (ORCADES), has already helped identify many regions of DNA influencing the amount of sugars found on Immunoglobulin G (IgG) antibodies.
ORCADES volunteers help understand sugar-coating of inflammation
In more recent work, Viking Health Study - Shetland volunteer data was used. It was key to investigating the glycosylation of transferrin - a protein involved in the transfer of iron to where it's needed in the body. For example, haemoglobin, a protein in red blood cells that helps carry oxygen to all cells and tissues, cannot perform its function without iron.
In addition, researchers also wanted to know whether the glycosylation of transferrin and IgG proteins is controlled by the same or different genes.
We could think of glycosylation enzymes as bakers and of proteins as baked goods. For example, some bakers specialise in only one style of baked goods, such as cakes or pastries. Similarly, researchers identified some enzymes specialised only in glycosylation of IgG and others specifically for transferrin. However, some versatile bakers can make both great cakes and pastries. Likewise, the authors also found that some enzymes can be involved in the glycosylation of both proteins.
How does this translate scientifically? Three glycosylation enzymes (or specialised bakers), called MGAT5, ST3GAL4 and B3GAT1, were linked with glycosylation of transferrin (pastries) only, while two different enzymes, MGAT3 and ST6GAL1 were associated uniquely with glycosylation of IgG (cakes). Finally, two further enzymes (versatile bakers), FUT6 and FUT8, were associated with the glycosylation of both proteins.
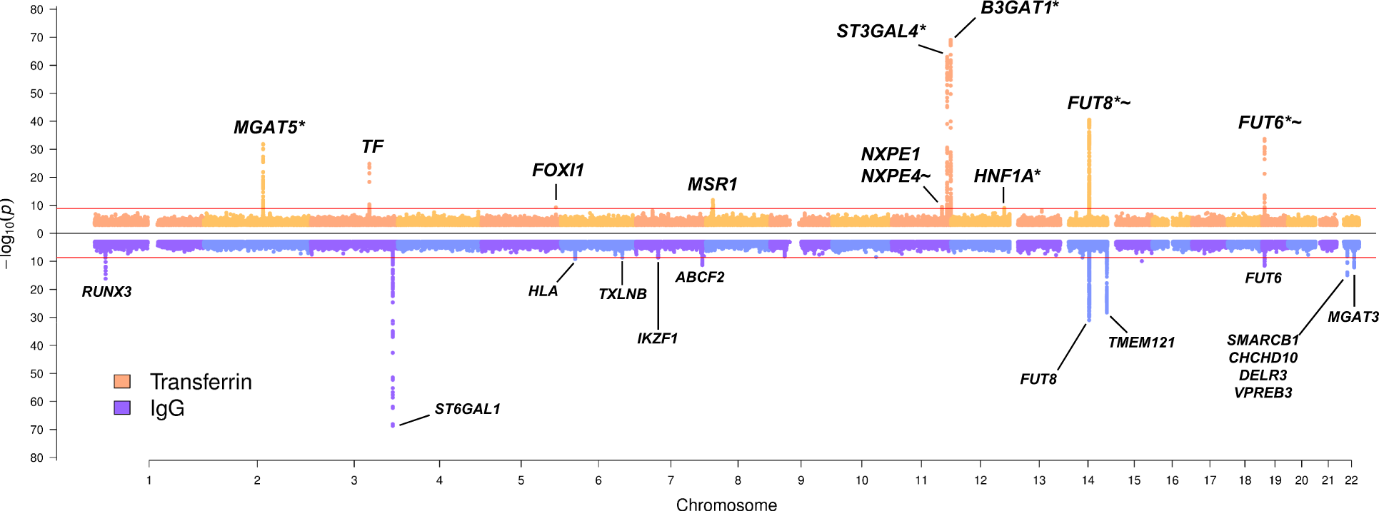
Taking it a step further, can versatile bakers use the same equipment when preparing cakes as they do with pastries? Unsurprisingly, the answer is, no. Depending on the baked goods, the same baker will use slightly different utensils. The same goes for the tools used in glycosylation. When looking at the shared associations at FUT genes (versatile bakers), researchers showed that the glycosylation process is driven by protein-specific variants (different utensils), where one variant affects the glycosylation of IgG and another that of transferrin
Overall, several other genes were linked with transferrin or IgG. This suggests that the control network of the glycosylation of each protein could be quite different. What does all this work mean for health research? By understanding the process of glycosylation and the enzymes involved, researchers can begin to understand its relationship with our health.
This work, published in Nature Communications, contributes a new piece to the extremely complicated jigsaw of glycosylation.
Genetic regulation of post-translational modification of two distinct proteins

