We wanted to investigate the possible link between COVID-19 vaccines and low platelet count, blood clotting or bleeding events, to provide urgently needed information on vaccine safety at a national scale.
Our research focused on people who received their first dose of either the Oxford-AstraZeneca or Pfizer-BioNTech vaccine in Scotland between 8 December 2020 and 14 April 2021.
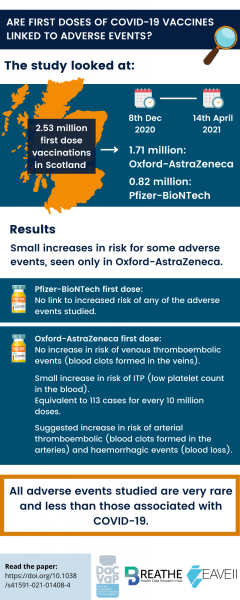
The study included 57.5% of the Scottish population, or 2.53 million people. Of these:
- 1.71 million people had the Oxford-AstraZeneca vaccine
- 0.82 million people had the Pfizer-BioNTech vaccine
- Less than 10,000 people had the Moderna (mRNA 1273) vaccine
We did not investigate the Moderna vaccine because the vaccinated population is too small to study rare events.
What adverse events were being studied?
We included four main types of adverse events in the study, based on patient reports. These are:
- Idiopathic thrombocytopenic purpura (ITP) – a low platelet count in the blood. Platelets form clots when blood vessels are damaged, preventing blood loss.
- Venous thromboembolic events – blood clots that form in the veins and/or break away from the clotting site, travelling through the veins.
- Arterial thromboembolic events – blood clots that form in the arteries and/or break away from the clotting site. In serious cases, this can result in heart attack or stroke.
- Haemorrhagic events – blood loss. This can be internal or external bleeding.
What methods were used in this study?
The study used two main methods to look at the data. These are known as an incident matched case control study and a self-controlled case series (SCCS) analysis.
In the incident matched case control study, every person found to have low platelet count or a blood clotting or bleeding event, after the first dose of a vaccine was paired with similar people who had not been affected. This method is used to check for any other possible causes of the adverse event.
Only people with no history of these events since 1 September 2019 were included as cases in the study. The matching was based on age by year, sex at birth, health history and residential area.
We checked all positive findings from the matched case control study using the SCCS method. This method calculates the risk of an adverse event for each affected person before and after vaccination, rather than comparing them to others.
What can we conclude from this study?
The study found that the Pfizer-BioNTech vaccine first dose has no link with increased risk of any of the adverse events listed above.
The first dose of the Oxford-AstraZeneca vaccine was linked to a small increased risk of ITP (low platelet count), equivalent to 113 cases for every 10 million doses. No increased risk was found for venous thromboembolic events after having the Oxford-AstraZeneca vaccine.
We found a suggestion of increased risk of arterial thromboembolic and haemorrhage events with the Oxford-AstraZeneca vaccine.
All of these events are very rare. The risk of them happening after vaccination is at a similar level to other common vaccines, including Hepatitis B, MMR, and Influenza.
Why is this research important?
This is one of the first real-world studies of rare low platelet count, blood clotting and bleeding events of all vaccinated people at a national level. Large studies like this are needed to identify rare adverse events and show whether they are likely to be linked to the vaccine. The data can be used to ensure safety in national vaccination programmes, especially ones that have prioritised by COVID-19 risk level.
What is next?
In this research, we looked at low platelet, blood clotting and bleeding events after the first dose of the two most commonly available vaccines in the UK. Because older and at-risk groups were prioritised for vaccines, there were less data for younger vaccinated people and those not thought to be at high risk of serious COVID-19 outcomes.
We plan to update our health data analysis as the vaccine programme continues. This will include younger groups, second doses, and newly approved vaccines like Moderna and Janssen.
Cite as
Simpson, C.R., Shi, T., Vasileiou, E. et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med (2021). https://doi.org/10.1038/s41591-021-01408-4
Note
This plain English summary and infographic were created with the support and feedback of the EAVE II Patient Advisory Group (PAG). This article in particular was reviewed by PAG members Sandra J and Eve S.
To learn more about the PAG, see Our EAVE II Patient Advisory Group (PAG) | The University of Edinburgh