Clinical trials at CRM
Find out about clinical trials currently being carried out at the Centre for Regenerative Medicine.
Our Centre conducts basic research into stem cell science, with a view to developing effective clinical treatments in the future.
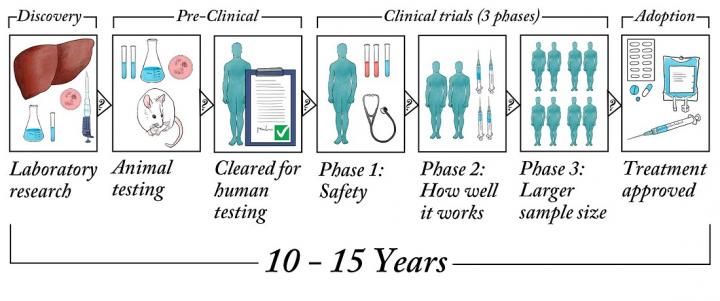
Developing new medical treatments is a long process with many steps to ensure safety and effectiveness. New ideas for treatments must first be developed and rigorously tested in laboratories before being tested on people in clinical trials.
The clinical trial approval process has many government rules and regulations to protect patients. If approved in clinical trials, a treatment then needs the pharmaceutical and biotech industries to further develop it for widespread use. Overall, making a new treatment can easily take 15 to 20 years.
Some of our programmes are starting to reach the clinical trial stage.

