A new biomarker analysis resource for clinical research
The British Heart Foundation Cardiovascular Biomarker Laboratory
The British Heart Foundation Cardiovascular Biomarker Laboratory is based in the Queen’s Medical Research Institute and is supported by the British Heart Foundation. The facility offers secure receipt, processing, archiving, shipment, and analysis of clinical samples. Secure sample storage is also available within this ACCORD accredited facility.
We participate in the UKNEQAS External Quality Assessment for Cardiac Biomarkers scheme (hsTnI and NT-proBNP) and offer a range of cardiac biomarkers and clinical chemistry assays on an Abbott ARCHITECT ci4100 Integrated Analyser. We obtained ACCORD accreditation in 2021 and can run locally sponsored clinical trial samples.
Our facility offers an efficient and accurate biomarker analysis service, with support from study design and planning through report and interpretation of the results. We provide robust reports on assay precision. Our strength is in the World-leading expertise in running high-quality clinical studies on a large scale and expertise in the analysis of Cardiac Biomarkers. So far, we have established partnerships with the R&D teams at Abbott Diagnostics, Siemens Healthineers and Roche Diagnostics and LumiraDx.
The BHF Cardiovascular Biomarker Laboratory offers the potential for academic and commercial collaboration with a wide range of biomarker assays.
Location and contact details of the facility:
Room W3.37, W3.38,
Centre for Cardiovascular Science,
Queen's Medical Research Institute,
47 Little France Cres, Edinburgh EH16 4TJ
Tel: 0131 242 26705
Email: CVS.biomarkers@ed.ac.uk, t.fujisawa@ed.ac.uk
Our research
Find out more about our research into High-Sensitivity Cardiac Troponin and Coronary Heart Disease.
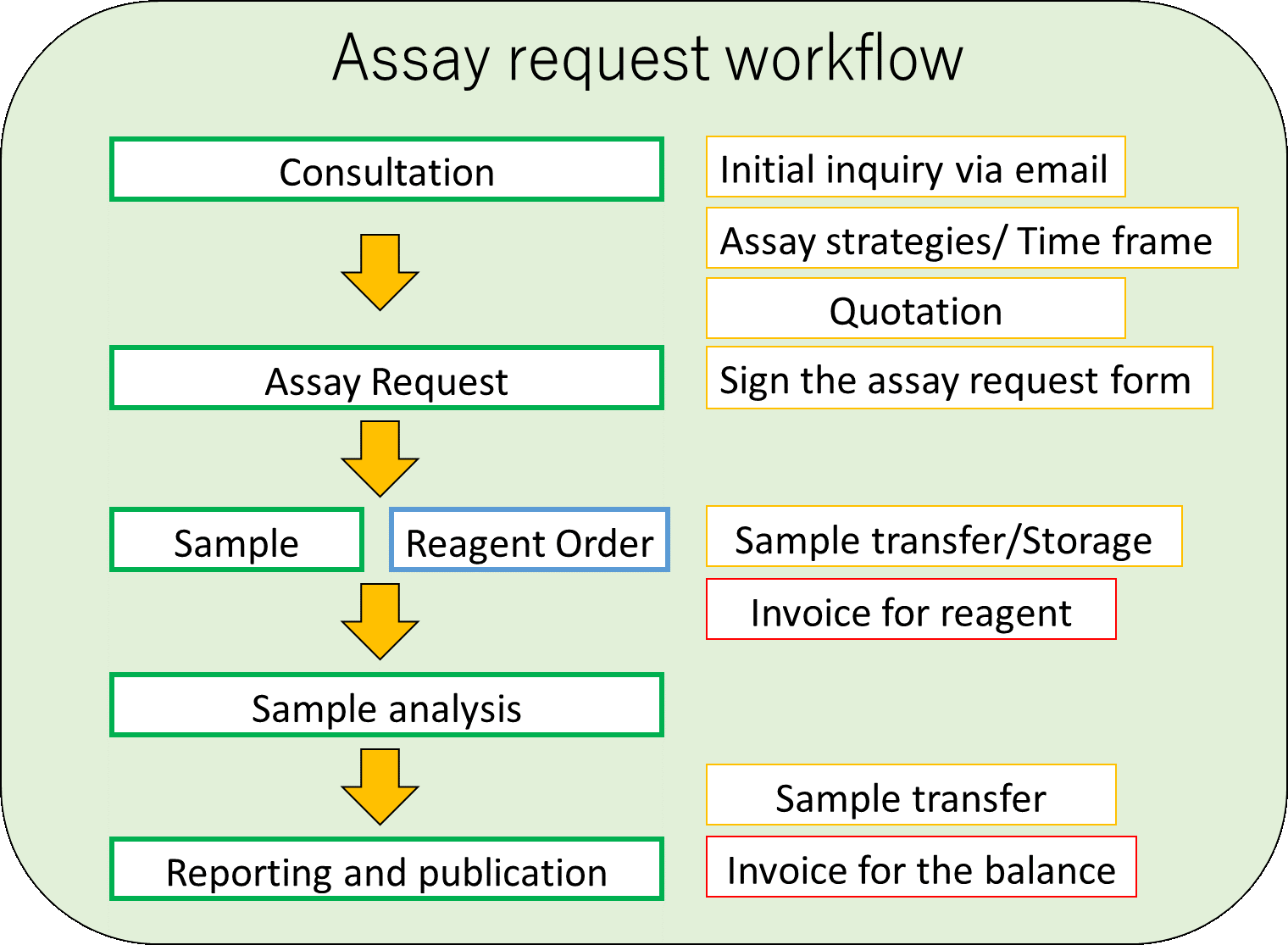
Assay Requests
Following the initial inquiry, we will discuss the assay strategies and the time frame.
We use the assay request form for the consultation to record your request. Required information includes assay menu, sample number and sample types, and time frame. Some assays require a specific sample type, and we can advise you on specific requirements.
Please contact us by email at CVS.biomarkers@ed.ac.uk for the first instance.
This article was published on



