Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland
May 2021: A milestone study, which points to the success of first dose mass vaccination in reducing COVID-19 hospitalisations across Scotland, is now available from The Lancet.
Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study
Vasileiou E, Simpson CR, Robertson C, Shi T, Kerr S, et al.
Available at The Lancet: https://doi.org/10.1016/S0140-6736(21)00677-2
Statistical Analysis Plans (SAPs) available: Protocol and analysis | The University of Edinburgh
Plain English summary
Background
The Pfizer-BioNTech (BNT162b2 mRNA) and Oxford-AstraZeneca (ChAdOx1) COVID-19 vaccines were tested in clinical trials. They were both found to give protection against COVID-19 infection. They are now being used to vaccinate the public in the UK and several other countries.
We know the vaccines worked in clinical trials, and now we need to know what this means in the ‘real world’. We wanted to know how effective the first dose of these vaccines is in preventing COVID-19 hospital admissions.
Why is this study important?
Pfizer-BioNTech said the second dose should be given at 3 weeks. Oxford-AstraZeneca said the second dose should be given between 4-12 weeks. In the UK, the second dose of all vaccines is given within 12 weeks of the first dose. This study is important for the Government who make the policy on when the vaccine doses are given. This is the first study to measure the impact of the UK’s vaccine strategy.
EAVE II data
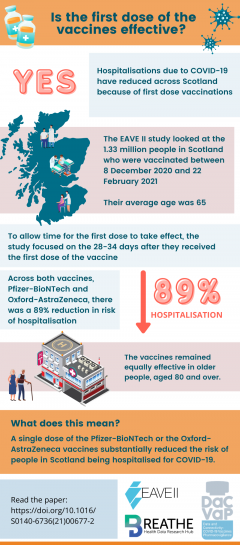
The EAVE II cohort is made up of data on vaccinations, primary care, COVID tests, hospitalisations and deaths for 5.4 million people in Scotland. This is around 99% of people living in Scotland.
What we did
We analysed the EAVE II data to find out the risk of hospital admission for COVID-19 after only having one dose of vaccine. We measured how many people had been admitted to hospital after being vaccinated.
What we found
Between December 8, 2020, and February 15, 2021, a total of 1,331,993 people were vaccinated. The average age of those vaccinated was 65 years.
By 28-34 days after being vaccinated:
The first dose of the Pfizer-BioNTech vaccine reduced the risk of a COVID-19 hospital admission by 91%
The first dose of the Oxford-AstraZeneca vaccine reduced the risk of a COVID-19 hospital admission by 88%
The first dose of either vaccine reduced the risk of a COVID-19 hospital admission by 83% in older adults aged 80 years and older. What does this mean? A single dose of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines reduced the risk of people in Scotland being admitted to hospital due to COVID-19.
These up-to-date findings show that first doses of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines protect against hospital admissions in all adult age groups including older age groups over 80. These findings highlight the need to speed up the roll-out of first vaccine doses and achieve high population coverage.
Additional information
Follow the link to meet the EAVE II team, the excellent researchers leading the study: Meet the EAVE II team | The University of Edinburgh
The study was funded by the Medical Research Council, the National Institute for Health Research and Health Data Research UK, and supported by the Scottish Government.
Additional support has been provided through Public Health Scotland and Scottish Government Director-General Health and Social Care, and the UKRI COVID-19 National Core Studies Data and Connectivity programme led by HDR UK.
Cite as
Vasileiou E, Simpson CR, Robertson C, Shi T, Kerr S, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. The Lancet. Published online April 23, 2021. doi: 10.1016/S0140-6736(21)00677-2
Note
This plain English summary and infographic were created with the support and feedback of the EAVE II Patient Advisory Group (PAG). This particular article was reviewed by PAG members Sandra J, Kamil S and Eve S.
To learn more about the PAG, see: Our EAVE II Patient Advisory Group (PAG) | The University of Edinburgh
Pre-print
Results from this study were originally released in a pre-print article.
A full summary of this work is also available on our website.


