Defining the interferon response during human cytomegalovirus infection
The Grey lab has applied fluorescent screens to explore the role of interferon (IFN) stimulated genes (ISGs) in viral infection.
IFNs are a crucial first line of defense against viral infection. They also shape the adaptive immune response by triggering release of cytokines and chemokines. Developing a better understanding of the interferon response and how viruses counteract its effects has important implications for how we treat viral infections and the development of vaccines.
Although human cytomegalovirus (HCMV) has evolved multiple strategies to subvert and inhibit antiviral effects of IFNs, evidence would suggest they still play a vital role in controlling replication and pathogenesis. Individuals with mutations in key IFN signalling genes are lethally susceptible to HCMV infections and recombinant IFN has been successfully used in treating congenital HCMV and HCMV infection in acquired immunodeficiency syndrome (AIDS) patients. Furthermore, murine CMV is more lethal in IFN knockout mice.
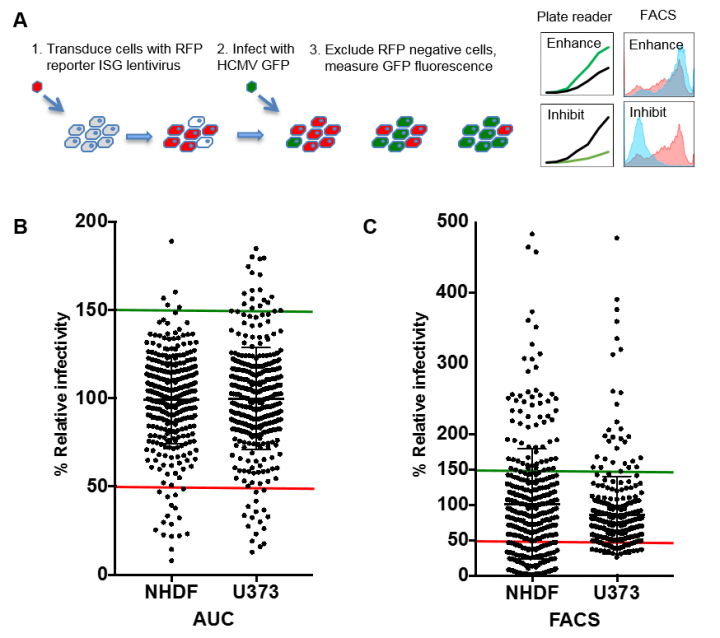
Systematic analysis using arrayed ISG expression libraries is an effective method for identifying key components of the IFN response. The screen relies on the use of reporter constructs expressing ISGs and Red Fluorescent Protein (RFP) in a high-throughput format. Transduction of cells with the lentivirus allows monitoring of ISG expression by RFP fluorescence. Cells are then infected with a green fluorescent protein (GFP) expressing virus and viral replication versus ISG expression is monitored by flow cytometry. ISGs that confer antiviral effects result in significant reductions in GFP signal among RFP positive populations. Previous screens have successfully identified multiple novel antiviral ISG candidates.
We have used this approach and have identified multiple ISGs with known of novel anti-HCMV activity. In addition we have performed parallel screens in IRF3 KO cells to identify ISGs that have a direct effect on HCMV replication, rather than an indirect effect through triggering the IFN pathway through over expression. We are currently characterising a number of hits from this screen that have important implications for how IFN regulates HCMV replication and how the virus has evolved strategies to circumvent the cellular defence mechanism.

