Innovations in genome modification in the chicken
Recent advances in gene editing technologies at the NARF will provide novel tools and models for basic, biomedical and agricultural research.
Compared with mammals, producing genetically altered (GA) birds has been more challenging due to the complex structure of the avian zygote (fertilized egg) and the organisation of the avian embryo. Early attempts to develop methods for genome engineering of the chicken were largely unsuccessful1,2, but a method using lentiviral vectors developed at Roslin enabled the creation of a number of genetically-modified chicken lines that are valuable tools for developmental biology3,4, avian immunology5 and biotechnology6,7. Yet the process to create a stable GA chicken line, in which all offspring would carry the introduced gene, continued to be time-consuming, requiring multiple crosses and generations of chickens. Other drawbacks were that the size of the transgene introduced was limited by the vector capacity, and precise modification of genes in the genome (gene editing) was not possible.
Several recent technological advances have improved the efficiency of genome editing in the chicken and consequently the creation of GA chicken lines. These advances include the refinement of the culture conditions for chicken primordial germ cells8, the development of chicken sterile surrogate hosts9,10 and new methods to create precise and targeted gene modifications10,11.
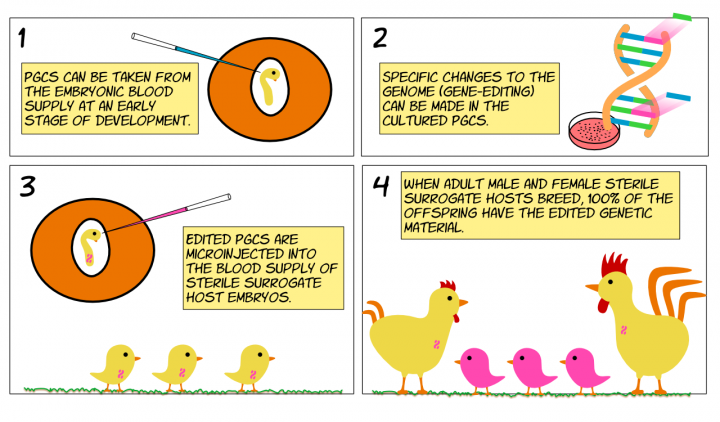
Primordial germ cells (PGCs) are stem cells that give rise to sperm or eggs. PGCs taken from developing chicken embryos can be grown in culture8 and modified in the laboratory by inserting a foreign gene (transgenesis)12 or introducing specific changes in the genome (editing)13. When edited PGCs are introduced into an embryo they have to compete with endogenous PGCs, resulting in variable and often low rates of chicks generated from the edited PGCs. To avoid this, sterile surrogate host chickens have been developed, in which the germ cell lineage of both males and females can be ablated after the introduction of a chemical compound10. The chemical ablation compound can be microinjected into the blood supply of a developing sterile surrogate host embryo at the same time as the altered PGCs10. The genetically altered PGCs migrate to the testes or ovaries, where they give rise to sperm or eggs once the animals mature. Mating of male and female sterile surrogate hosts results in 100% transmission of introduced PGCs in the first generation of offspring10. Compared to previous methods, these advances reduce the number of animals used to create a GA chicken line14 and reduce the time required to create a GA chicken line from about two years to less than one year. Consequently, the costs associated with producing a new GA chicken line are reduced.
These major improvements in genome engineering technologies in the chicken, led by Dr Mike McGrew, combined with the opportunities provided by using CRISPR/Cas9 technologies, enable the rapid and cost-effective production of GA chicken lines.
The potential of gene editing in chickens

The chicken is an important agricultural animal both in the UK and around the world. Genome engineering technologies can improve poultry welfare and research conducted with the NARF has enabled the introduction of beneficial traits10 and disease resistance7,15 in agriculturally important chicken breeds.
Gene editing in chickens has the potential to advance our knowledge in a wide range of research fields, including avian immunology. The creation of immune gene reporter4,5 and knock-out lines will advance our understanding of the avian immune system, which is poorly understood compared to mammals. The NARF has a Specified Pathogen Free (SPF) facility that now has the capability to generate transgenic or gene edited chickens under SPF conditions. This will aid research that aims to understand how the avian immune system responds to infection, facilitating the design of effective vaccines.
Recently, the NARF’s gene editing technologies have made possible research investigating avian sex determination using targeted mutations in the DMRT1 gene11, and the creation of layer chickens with introduced feather traits that are potentially beneficial in tropical climates10. Gene editing in the chicken will soon be used to explore extra-retinal photoreceptors in birds16 and the genes responsible for defects of the optic fissure closure in the eye that cause ocular coloboma17.
*The NARF is supported by a BBSRC strategic funding award.
Useful Links
Sterile surrogate host chicken lines; iCaspase9 and DDX4 KO
Genome engineering facilities at the NARF
Research conducted using the NARF's resources
Publications
- Sang, H. Prospects for transgenesis in the chick. Mechanisms of Development 121, 1179–1186 (2004).
- Love, J., Gribbin, C., Mather, C. & Sang, H. Transgenic birds by DNA microinjection. Bio/Technology 12, 60–63 (1994).
- McGrew, M. J. et al. Efficient production of germline transgenic chickens using lentiviral vectors. EMBO Rep. 5, 728–733 (2004).
- Davey, M. G., Balic, A., Rainger, J., Sang, H. M. & McGrew, M. J. Illuminating the chicken model through genetic modification. Int. J. Dev. Biol. 62, 257–264 (2018).
- Balic, A. et al. Visualisation of chicken macrophages using transgenic reporter genes: Insights into the development of the avian macrophage lineage. Development 141, 3255–3265 (2014).
- Herron, L. R. et al. A chicken bioreactor for efficient production of functional cytokines. BMC Biotechnol. 18, 82 (2018).
- Lyall, J. et al. Suppression of avian influenza transmission in genetically modified chickens. Science. 331, 223–226 (2011).
- Whyte, J. et al. FGF, insulin, and SMAD signaling cooperate for avian primordial germ cell self-renewal. Stem Cell Reports 5, 1171–1182 (2015).
- Taylor, L. et al. Efficient TALEN-mediated gene targeting of chicken primordial germ cells. Development 144, 928–934 (2017).
- Ballantyne, M. et al. Direct allele introgression into pure chicken breeds using Sire Dam Surrogate (SDS) mating. Nat. Commun. 12, 659 (2021).
- Ioannidis, J. et al. Primary sex determination in birds depends on DMRT1 dosage, but gonadal sex does not determine adult secondary sex characteristics. Proc. Natl. Acad. Sci. 118, e2020909118 (2021).
- McGrew, M. J. et al. Localised axial progenitor cell populations in the avian tail bud are not committed to a posterior Hox identity. Development 135, 2289–2299 (2008).
- Macdonald, J. et al. Efficient genetic modification and germ-line transmission of primordial germ cells using piggyBac and Tol2 transposons. Proc. Natl. Acad. Sci. U. S. A. 109, 8803 (2012).
- Panda, S. K. & McGrew, M. J. Genome editing of avian species: implications for animal use and welfare. Lab. Anim. 002367722199840 (2021). doi:10.1177/0023677221998400
- Long, J. S. et al. Species specific differences in use of ANP32 proteins by influenza A virus. Elife 8, (2019).
- Meddle, S. L., Dunn, I. C., McGrew, M. & Perez, J. H. Light in early life: understanding the mechanisms of embryonic photoreception to improve poultry welfare and production. Funder: UK Research and Innovation Available at: https://gtr.ukri.org/projects?ref=BB%2FV001981%2F1.
- Rainger, J. Novel approaches to define tissue fusion mechanisms in embryonic development. Funder: UK Research and Innovation Available at: https://gtr.ukri.org/projects?ref=MR%2FS033165%2F1.
Graphics: Lindsay Henderson and Alec McEachran at Science Doodles


