Fighting infectious diseases
Tackling the leading cause of losses in livestock.
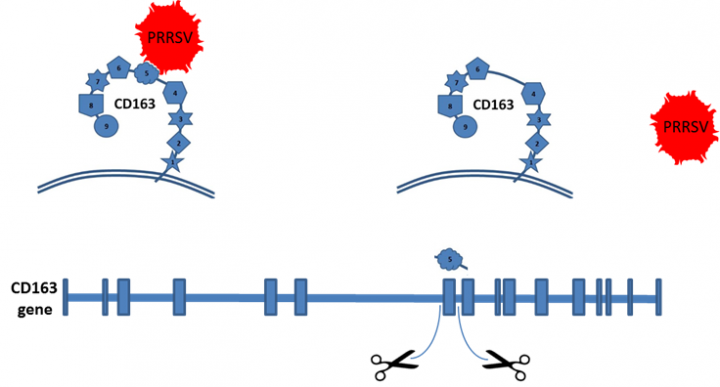
Porcine Reproductive and Respiratory Syndrome virus (PRRSV)
PRRSV causes abortion in pregnant sows, death of new-born piglets and a failure to thrive in older animals. As a global disease PRRSV is considered to have the highest impact on pork production, with an estimated annual productivity loss of €1.5 billion in Europe alone. PRRSV infects porcine macrophages through interaction with CD163 on the surface of the cell. We have used genome editors to remove a section of the CD163 protein to which the virus would normally bind, thereby rendering the virus incapable of infecting our pigs.
Collaboration: Dr. Christine Tait-Burkard
Papers: Burkard et al 2018, Burkard et al 2017
Africa Swine Fever (ASF)
ASF virus infects pig species such as warthogs and bush pigs that are endemic to sub-Saharan Africa, without causing significant pathology. In recent years this virus has become established in Eastern Europe where it causes a fatal haemorrhagic disease in domestic pigs. We have identified genetic variation between the warthog and domestic pig that could underlie the different responses of the two species, and used genome editors to introduce these changes to our pigs.
Paper: Lillico et al 2016, Lillico et al 2013
Influenza
Influenza is a zoonotic disease with financial and welfare impacts across the poultry and pig industries. All types of influenza virus that infect pigs are termed Swine Influenza Virus (SIV). Based both on the impact to the livestock industry and the ability to cause pandemics in humans, we focus on influenza A. Disease in pigs although associated with low mortality, results in poor growth and weight loss, and can cause abortion in pregnant sows. Since pigs can be infected by both avian and mammalian viruses, they represent a ‘mixing vessel’ for influenza reassortment. Currently, we are evaluating strategies to reduce viral attachment without compromising the immune responses generated by the surface receptors, especially in the upper respiratory tract.
Collaboration: Dr. Bhanu Telugu, University of Maryland
Awards: NIH-NIFA grant
Foot and Mouth Disease virus (FMDV)
Costing an estimated £5-16 billion each year, FMD is a highly infectious disease of cloven hooved mammals caused by FMDV; a member of a group of small RNA viruses called the Picornavirus family. The FMDV genome encodes for 4 structural proteins (VP1-4) which form the viral capsid. One of these structural proteins, VP1, contains a conserved Arg-Gly-Asp (RGD) motif that interacts with RGD-binding integrins such as integrin αVβ6 during viral entry. We are currently investigating whether host-variation within the RGD-binding integrins effects viral infection in vitro or whether the differences in susceptibility arise at a different stage of the viral replication cycle.

