Liz Patton Research Group
Targeting developmental cell states in melanoma

Research in a Nutshell
Melanocyte Development and Melanoma
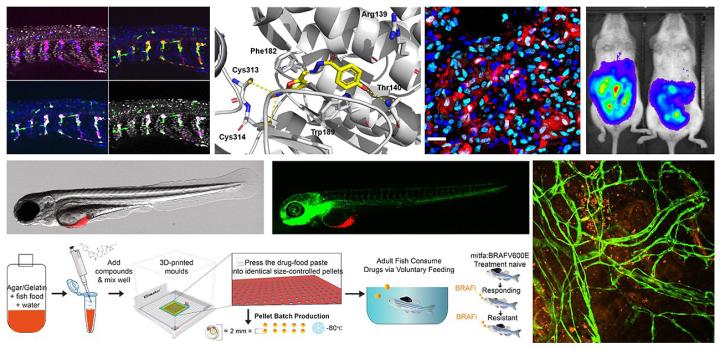
Melanoma accounts for 80% of the deaths from skin cancer, and incidence continues to rise rapidly. Aggressive and resistant to chemotherapies, individuals with metastatic melanoma often have a life expectancy of less than one year. Our research is focused on understanding how melanocytes – the pigment cells that become melanoma – develop, divide, migrate and maintain homeostatis within their microenvironment, as well as the genetic and cellular events that cause melancoytes to form moles and their progression to invasive cancer. To do this, we use the zebrafish system, which allows both the visualization of developing and migrating melanocytes, as well as their aberrant progression to melanoma.
The zebrafish is a powerful model system to study developmental biology, chemical biology and disease models. Due to the similar genetic, molecular and cancer pathology between humans and fish, our melanoma progression model can be viewed as an important starting point for identifying novel genes, environmental conditions, and therapeutic compounds that affect melanoma progression.
Our lab uses the zebrafish system to understand the development of melanocytes, with a view to how these processes are altered in melanoma. We use genetics and chemical-biology to discover the fundamental processes that contribute to melanocyte development during embryogenesis, and explore how these processes contribute to melanoma development. We have two zebrafish facilities at the IGMM, and access to a wide range of transgenic and genetic lines, diverse chemical libraries, and state-of-the-art imaging facilities.

People |
|
| Professor E. Elizabeth Patton | Group Leader |
| Kelly Blacklock | Veterinary Clinical Research Fellow |
| Alessandro Brombin | Research Fellow |
| Hannah Brunsdon | Research Fellow |
| Jana Travnickova | Research Fellow |
| Adelaide Young | Research Fellow |
| Yuting Lu | Research Fellow |
| Sarah Muise | PhD student |
| Stephanie MacMaster | PhD student |
| Oscar Jackson | PhD student (joint PhD student with Martin Taylor) |
Contact
Publications
- Yuting Lu, Jana Travnickova, Mihaly Badonyi, Florian Rambow, Andrea Coates, Zaid Khan, Jair Marques, Laura Murphy, Pablo Garcia-Martinez, Richard Marais, Pakavarin Louphrasitthiphol, Alex Von Kriegsheim, Joseph A Marsh, Valeria Pavet, Owen J Sansom, Robert Illingworth. ALDH1A3-acetaldehyde re-wires neural crest stem cell and high metabolism states to potentiate melanoma heterogeneity. bioRxiv https://doi.org/10.1101/2023.10.28.564337
- Brunsdon H, Brombin A, Peterson S, Postlethwai JH, Patton EE. Aldh2 is a lineage-specific metabolic gatekeeper in melanocyte stem cells. Development 2022 PMID: 35485397
- Brombin A, Simpson DJ, Travnickova J, Brunsdon H, Zeng Z, Lu Y, Young AIJ, Chandra T, Patton EE. Tfap2b specifies an embryonic melanocyte stem cell population that retains adult multi-fate potential. Cell Reports, 2022. PMID: 35021087.
- Johansson JA, Marie KL, Lu Y, Brombin A, Santoriello C, Zeng Z, Zich J, Gautier P, von Kriegsheim A, Brunsdon H, Wheeler AP, Dreger M, Houston DR, Dooley CM, Sims AH, Busch-Nentwich EM, Zon LI, Illingworth RS, Patton EE. PRL3-DDX21 Transcriptional Control of Endolysosomal Genes Restricts Melanocyte Stem Cell Differentiation. Developmental Cell, 2020. PMID:32652076.
- Travnickova J, Wojciechowska S, Khamseh A, Gautier P, Brown DV, Lefevre T, Brombin A, Ewing A, Capper A, Spitzer M, Dilshat R, Semple CA, Mathers ME, Lister JA, Steingrimsson E, Voet T, Ponting CP, Patton EE. Zebrafish MITF-Low Melanoma Subtype Models Reveal Transcriptional Subclusters and MITF-Independent Residual Disease. Cancer Research, 2019. PMID: 31582381.
Full publication list can be found on Research Explorer: Elizabeth Patton — University of Edinburgh Research Explorer

Collaborations
-
Tamir Chandra (MRC Human Genetics Unit)
-
Chris Ponting (MRC Human Genetics Unit)
-
Colin Semple (MRC Human Genetics Unit)
-
Martin Taylor (MRC Human Genetics Unit)
-
Robert Semple (The University of Edinburgh)
-
Veronica Kinsler (Crick Institute)
-
Richard White (MSKCC, USA)
Partners and Funders
- Medical Research Council
- Melanoma Research Alliance
- Rosetrees Trust
- CLOVES Syndrome Community & Chan Zuckerberg Initiative
- European Research Council
Scientific Themes
Melanoma, Melanocyte, Zebrafish, Disease models, Chemical genetics
Technology Expertise
Chemical biology, Drug target ID, CRISPR mutations


