Research
Our starting point is the discovery of human disease genes. The major focus of our research is then understanding the cellular and molecular functions of the proteins they encode.
Using a ‘human as a model organism’ approach we aim to gain insights into:
- how cell number is determined during development to explain the range of size in man and other mammals
- the modalities by which DNA damage activates innate immune pathways to counter cancer
- the consequences of embedding ribonucleotides into the nuclear genome
We work with individuals with microcephalic primordial dwarfism, a disorder of extreme growth restriction. We also study Aicardi-Goutières syndrome, a paediatric inflammatory disorder that mimics congenital viral infections such as Zika, CMV and Rubella. From this our research has developed two main themes:
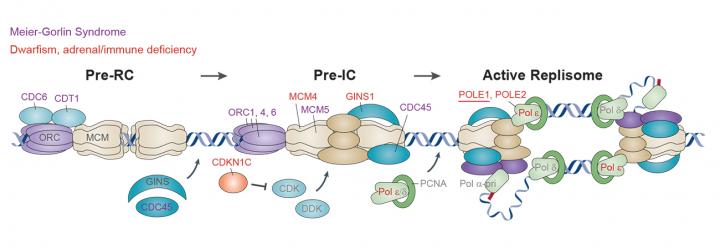
- Disorders of size: understanding how DNA replication and repair, along with epigenetic regulators, establish organ and organism size.
- Genome-embedded ribonucleotides: investigating their role in genome instability, mutagenesis and inflammation.
Adapted by permission from Macmillan Publishers: Replication Stress and Cancer, Gaillard et al. Nature Reviews Cancer, copyright 2015.
Disorders of size: organism growth
The greatest difference between mammals is size, with 8 orders of magnitude difference between the smallest and largest. Also, a defining feature of humans is the evolutionary expansion of the cerebral cortex of the brain. While developmental patterning has been defined in exquisite detail, much remains to be learnt about the developmental and evolutionary factors controlling organ and organism size.
Through studies on extreme growth disorders, we have identified 20+ genes that regulate cerebral cortex volume and organism size. The majority encode key components of cellular machinery controlling cell proliferation, which consequently impact on cell number generated during development. Recently we have also discovered an epigenetic regulator that could determine human size through modulating cell fate decisions.
Supported by an ERC Advanced Grant award, we are using whole genome sequencing to identify further microcephalic primordial dwarfism genes. Alongside, we are pursuing cell and developmental studies to define pathogenic mechanisms and further understanding of mammalian growth regulation.
Genome-embedded ribonucleotides: DNA damage and inflammation
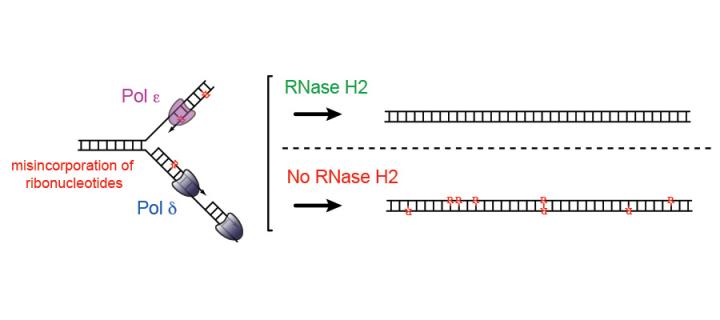
Our information store, the genome, is a polymer formed of deoxyribonucleotides. It is consequently surprising that ribonucleotides are the most common aberrant nucleotides within the mammalian genome. We have established that over a million ribonucleotides are incorporated into the genome of every replicating cell. They are normally removed by RER (ribonucleotide excision repair), a repair pathway that acts alongside MMR/BER/NER to ensure genome integrity.
RER is initiated by Ribonuclease H2, an enzyme encoded by the three genes most commonly mutated in Aicardi-Goutières syndrome (AGS). AGS is a monogenic autoinflammatory disorder that mimics in utero viral infections such as Zika, CMV and Rubella.
We have found embedded ribonucleotides to be a therapeutically-relevant source of endogenous PARP-trapping lesions. We have harnessed genome-embedded ribonucleotides as a tool to track replicative polymerases, showing that lagging-strand replication elevates mutation rates at protein binding sites in eukaryotic genomes.
We have also studied genome instability arising from dysfunction of this key genome stability enzyme and resulting in activation of innate immune pathways. This has led us to discover micronuclear DNA as an important source of immunostimulatory DNA in the cytosol.
This link between genome instability and innate immunity is also relevant to cancer, both with regards to the early stages of neoplastic transformation and for cancer treatment.


