Empowering development of treatments for COVID-19
The QMRI COVID-related Research Hub is a rapid response to the COVID-19 pandemic, bringing together experimental medicine clinical studies, interdisciplinary research teams, industrial partners and academic collaborators to understand the mechanisms of disease and discover effective treatments.
Protecting and cultivating internationally-leading research facilities and opportunities
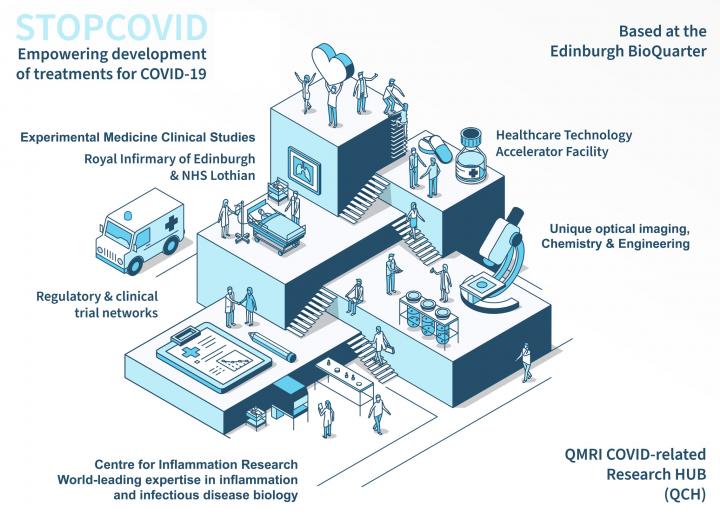
The QMRI COVID-related Research Hub (QCH) has been established as a rapid response to the current global COVID-19 pandemic, repurposing facilities and expertise to continue essential world-class biomedical pre-clinical research. It is positioned to take forward a vital programme of research from the laboratory to experimental medicine clinical studies, and then forward to treatments for patients.
The QCH integrates the uniquely-relevant research portfolio of the Centre for Inflammation Research (CIR) with NHS Lothian, Edinburgh Royal Infirmary and University of Edinburgh clinical research teams, alongside the regulatory expertise of the Healthcare Technology Accelerator Facility and clinical trial networks. This all takes place on one integrated site, the Edinburgh BioQuarter.
QCH is part of the University of Edinburgh's College of Medicine and Veterinary Medicine.
Centre for Inflammation Research website
Healthcare Technology Accelerator Facility website
College of Medicine and Veterinary Medicine website
A new programme of internationally-leading COVID-19 research
STOPCOVID is an extensive new cross-sector, cross-discipline, experimental medicine research programme aimed at developing and re-purposing treatments for COVID-19 patients. Through this research we will aim to reveal and target the mechanisms controlling the harmful lung injury, inflammation and failure of repair that occur in severe COVID-19 disease.
We will operate across sectors; including funding from the medical research charity LifeArc, collaborations with international pharmaceutical companies, and direction and input from the Medicines and Healthcare products Regulatory Agency (MHRA) to help expedite the process of drug evaluation.
The programme combines scientific disciplines, from inflammation biology and chemistry to engineering and experimental medicine clinical studies. This will allow us to attempt to change the course of the disease and prevent the lung damage in patients with COVID-19 that leads to respiratory failure. This has the potential to reduce the need for ventilators and save lives. Discoveries will be immediately fed into national and global clinical trials networks, including RECOVERY, and to our partners.
Medicine and Healthcare products Regulatory Agency website
STOPCOVID combines unique local strengths to tackle COVID-19
Current evidence suggests that the severe and lethal lung disease associated with COVID-19 involves a dysregulated local inflammatory response, resulting in tissue injury and dysfunctional repair processes. On this basis, using knowledge of these pathways, this damage can be stopped at an early stage.
The STOPCOVID research programme draws upon 30 years of ground-breaking discoveries from the Centre for Inflammation Research; studying inflammation in health and disease to develop novel diagnostics and therapeutic approaches. The Centre's clinical and fundamental science researchers possess exquisitely relevant expertise for this research, translating scientific discoveries into real world impacts. This includes:
- regulation and therapeutic manipulation of inflammatory pathways
- unique lung imaging platforms
- clinical experimental medicine
- conducting clinical studies
The programme will approach the COVID-19 pandemic challenge as follows.
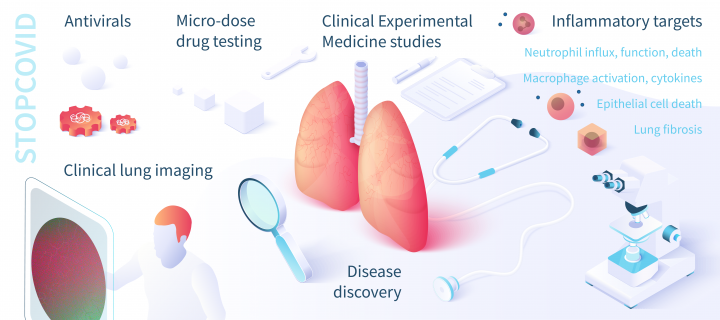
Disease and drug discovery
To fully understand COVID-19 disease we will collect patient samples, tissue and clinical data from patients in primary care and in hospitals, both with local protocols and in co-ordination with the UK-wide GenOMICC and ISARIC 4C (Clinical Characterisation of COVID-19 Consortium) studies. This will provide evidence for the best approaches to prevent the damaging inflammatory pathways that lead directly to lung injury. It will also enable laboratory testing of large collections of new and existing drugs that can target these pathways.
Rapid early phase experimental medicine studies
Based on expert knowledge of the pathways controlling inflammation, we will undertake clinical studies using the most promising drug candidates from existing clinical trials (including from collaborating pharmaceutical companies), and repurposed drugs - those already in use in humans for other conditions - that can modify these inflammatory pathways. Studies will be performed in primary care and/or in the hospital setting and include imaging in patient's lungs, in real time, to see the effects of drugs delivered. Findings will be fed into the UK national framework provided by the ISARIC 4C Consortium and the RECOVERY adaptive trial platform.
World-leading expertise in inflammation and infectious disease biology
The Centre for Inflammation Research has a wide range of candidate approaches directly relevant to the pathology of COVID-19, arising from extensive expertise in modifying innate antiviral pathways, cell death mechanisms, potentially harmful functions of inflammatory cells and inflammatory signals, and mechanisms of repair, with targets including:
- Neutrophils
- Macrophages
- Epithelial cells
- Endogenous antiviral agents
- Pro-fibrogenic pathways
Neutrophils are recruited to the lung in both humans and primate models with the related coronavirus infections SARS and MERS-CoV, and in severe cases are associated with lung injury and diffuse alveolar damage. This is a common pattern in other causes of ARDS (Acute Respiratory Distress Syndrome). Cell influx, signalling and death can be targeted.
Macrophages are prominent in disease, with local macrophage activation in the lung, high level production of ferritin and IL-6 observed in severe cases of COVID-19, and with potential similarities to macrophage activation syndrome, which frequently has a viral trigger. Cell function and signalling can be targeted.
Extensive death of the airway lining epithelial cells has been observed in severe cases of COVID-19, damaging the integrity of the lung and indicating dysregulated cell deaths pathways, such as apoptosis, necrosis or pyroptosis. Cell death pathways can be targeted.
Innate endogenous antiviral agents, such as host defence peptides have antiviral activity against lung viruses, including influenza and respiratory syncytial virus. Pathways which induce lung cell production of protective peptides can be targeted as a form of immunotherapy.
The Centre has extensive experience in fibrogenic pathway research and modifying harmful lung fibrosis in response to lung damage, with a record of translation from initial fundamental studies to targeted clinical application.
Unique optical imaging technologies
Within the Centre for Inflammation Research, the Translational Healthcare Technologies Group have developed a unique toolbox of optical sensor probes, including developments in partnership with Bath and Heriot Watt Universities. These can be used to see inside patients' lungs, in real time, using endomicroscopy techniques. This allows us to monitor how inflammatory pathways are being affected by the disease process and by possible treatments. We can use these technologies to deliver vital information on the mechanisms that control what is happening inside a COVID-19 patient's lungs, but also to test and observe new treatments in patients.
The unparalleled combination of the QCH research expertise and platforms, with its proximity to the Royal Infirmary of Edinburgh (a 1000 bed hospital which has now reconfigured into a COVID-19 'hot site' with large clinical trial capability), aligned with integrated regulatory expertise and clinical trial networks, and international pharmaceutical company collaborations, will support the rapid adoption of experimental medicine clinical studies for COVID-19, and for future pandemic viral infections, in addition to other respiratory failure or acute respiratory distress syndrome (ARDS) more broadly.
Donate or fundraise for us
The University of Edinburgh has a proud tradition of philanthropic support and community fundraising.
Private donations can have a profound impact on scientific research.
To learn more about fundraising for our STOPCOVID research programme, in the first instance please contact:
Chloe Kippen: Director, Advancement and Health Philanthropy, The University of Edinburgh: chloe.kippen@ed.ac.uk

