Translational Healthcare Technologies (THT)
Embracing team science across multiple disciplines to enable the rapid development and translation of novel healthcare technologies
About THT

The THT fuses scientific discovery with clinical translation and aims to challenge current human discovery, diagnostic and therapeutic pathways. We firmly believe in the human experimental model and placing the patient at the centre of the pathway.
The THT group is based within the Centre for Inflammation Research with affiliated group members from other leading UK Universities and UoE/NHS departments.
Their core focus is developing novel strategies to characterise and perturb inflammatory pathways in human disease. This precision medicine approach intersects across disciplines to advance sensing, imaging, therapeutic delivery and experimental medicine. To date, disease modelling and toxicology have been focused on preclinical models with limited success.
The THT team have developed pathways utilising primary human cells, tissues, organs and humans as the experimental model. Having conducted multiple First-in-Human studies to test the performance and safety of therapies, imaging probes and medical devices, THT has developed strong working relationships with the MHRA and clinical Sponsors to expedite translational pathways from concept to clinic. The THT team have developed pathways utilising primary human cells, tissues, organs and humans as the experimental model. Having conducted multiple First-in-Human studies to test the performance and safety of therapies, imaging probes and medical devices, THT has developed strong working relationships with the MHRA and clinical Sponsors to expedite translational pathways from concept to clinic.

THT champions experimental medicine with the group incorporating professional managerial/regulatory capabilities aligned with GMP accredited facilities (API and sterile fill) and robust Quality Management Systems (QMS) to fully support the translation from bench to bedside and back again.
Our key area of clinical and translational expertise is in pulmonary disease. In particular defining and intersecting acute and chronic pulmonary inflammatory diseases (pneumonia, Idiopathic Pulmonary Fibrosis (IPF), lung cancer, ARDS) to deliver patient benefit.
This group has developed a highly effective model to expedite the commercialisation and implementation of healthcare technologies for patient benefit and commercial success. Multiple funding sources, including UKRI, Wellcome and the US-based CARB-X fund, have supported the integration of physical scientists, biomedical scientists and clinicians into the same environment (in the basement of QMRI) and provides the requisite infrastructure (in term of expertise and facilities) to accelerate commercialisation. In doing so it removes the barriers that frequently preclude the development and translation of new healthcare technology.
Development of new healthcare technologies, including devices, molecules and software, will enable more effective, faster approaches to diagnosis, treatment and prevention of disease. Integration with emerging technologies such as sensors and digital advances are transforming medicine and social care and will increasingly provide disruption to traditional pathways of innovation in healthcare diagnosis and treatment pathways. Research and commercialisation in this sector is booming across the globe. In this new era a critical challenge is to accelerate the routes to move such innovations from research into commercial development and availability for use. The model for THT is incubation and acceleration of early commercial translation, with a focus on start-ups and entrepreneurship, embedding these skills and groups alongside the interdisciplinary academic innovation groups.
Our Current Work
The projects ongoing within the THT group span a range of scientific disciplines and stages of development; from fundamental discovery research through to experimental medicine clinical studies and beyond. Our work is supported by a range of funders, including UKRI, charities, academic-industrial partnerships and non-commercial and philanthropic funders. Our current collaborations involve more than 20 HEIs and over 70 external companies/organisations.
Current Pre-Clinical Research Projects
Molecular Alveoscopy
A major thrust of our work has been to develop novel and cutting edge tools to delineate pathophysiology and deliver drugs/therapies to the distal lung. This programmatic work brings together a range of disciplines and translational programmes including:
- Development of Novel Molecular Imaging: We are designing, manufacturing and evaluating new fluorescent molecules (‘SmartProbes’) that can be used to characterise a range of distal lung diseases including pneumonia, acute respiratory distress syndrome (ARDS), pulmonary fibrosis and lung cancer. We are working on SmartProbes for detection of neutrophilic, monocyte and macrophage activation, MMP activity, epithelial senescence, T cell activity and bacterial detection. To date we have translated 5 novel SmartProbes into in-patient studies and are now planning larger multi-site validation studies.
- Miniaturised Multifunctional Optical Fibre-Based Technologies: In partnership with the University of Bath, we are designing and manufacturing interventional medical devices and fibre technology for use in disease diagnosis and treatment, with an initial focus on pulmonary infection/inflammation and non-small cell lung cancer.
- Endoluminal Robotic Technologies for Interventional Pulmonology: In partnership with the School of Informatics (SoI) and the Edinburgh Centre for Robotics, we are developing bedside autonomous multimodal robotic systems for guidance and precision sampling for critically ill mechanically ventilated patients alongside systems enabling lung biopsy and theranostics (the detection and treatment of lung cancer as part of the same procedure).
- Industrial-Academic Partnerships: Evaluating drug-target engagement in the distal lung.
- Data Science and Inference: Using data-driven approaches to classify imaging and spectroscopy data, we are developing new algorithms that interface with interventional medicine tools. In partnership with SoI, we have established a cadre of software engineers, signal processing, machine learning and external industrial expertise.
- Deep Ultraviolet Light Therapies (U-Care): We are developing technologies capable of generating light in the deep UV part of the spectrum (190-225nm) and delivering it to previously poorly-accessible regions of a patient’s body, such as catheters, endotracheal tubes, surgical wound sites, the distal lung and in neurosurgery. Deep UV light can be used, alongside the precise surgical removal of infected tissue, to treat antibacterial infection in a way that does not lead to the emergence of antibiotic-resistant strains.
- Frugal Innovation for Diagnosis and Treatment of Infections in Low and Middle Income Countries: In collaboration with the Aravind, the world’s largest Eye Care System(India) and the Globalsurg network, we are developing low-cost systems that can detect and treat infections in, eye, skin and surgical settings.
-
Pre-clinical Testing: we have developed several translationally relevant model systems optimised for clinical tractability and imaging in humans. These include:
- Freshly-excised human lung tissues (from non-small cell lung cancer and IPF),
- Human lungs (obtained a UK wide consortium and deemed not fit for transplant) which can be ventilated and perfused on an ex-vivo lung perfusion system (EVLP)
- Tissue derived from cull-stock animals (therefore in keeping with the principles of the 3 R’s in animal research).
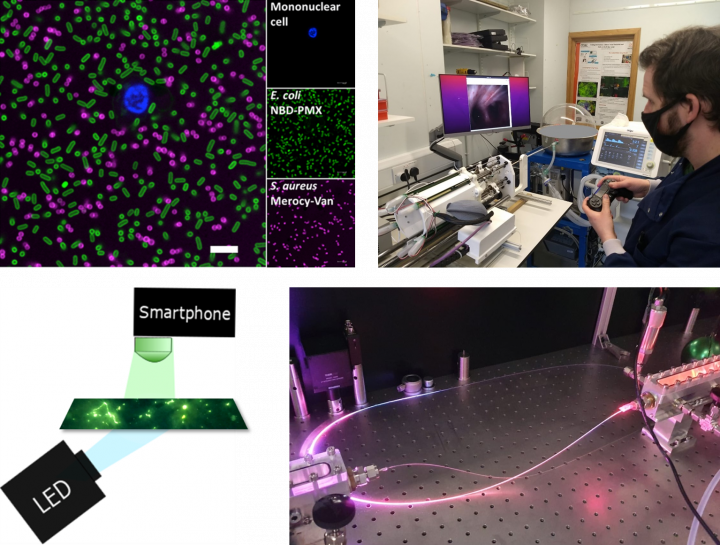
Highlights of Current THT Pre-clinical Research Projects: (Clockwise from Top Left) Bacterial labelling with novel fluorescent SmartProbes; Prototyping robotic technologies; Guiding light down an optical fibre; Low-cost system for diagnosis of eye infection.
Current Clinical Research Projects
The THT group are leading multiple clinical studies (observational and early phases) in the field of experimental medicine. These studies aim to progress immunoinflammatory modulation and bedside interventional experimental medicine.
STOPCOVID
STOPCOVID is a rapid interception experimental medicine programme for COVID-19. As a part of STOPCOVID, the THT group is currently undertaking several clinical studies that aim to progress therapeutic assets in early phase studies to evaluate safety of potential COVID-19 therapies:
1. DEFINE: This platform study is assessing the safety and PK/PD of potential COVID-19 therapies in small numbers of COVID-19-positive patients. This data may then support further development and larger scale trials. We are also assessing the effect of treatments on immune activation and clinical outcomes.
At present the study is assessing the efficacy of two candidate therapies:
- Nafamostat: an antiviral currently used to prevent blood clotting, and which may block entry of SARS-CoV-2 into host cells.
- TD139: an inhibitor of Galectin-3 previously shown to reduce inflammation in patients with idiopathic lung fibrosis.
The efficacy of both therapies will be assessed relative to current standard care. Further study arms, including a T cell therapy, inhaled antivirals and several immune cell modulators are in the pipeline and will be added to the DEFINE platform in the near future.
More details can be found on the DEFINE study website:
2. SPIKE-1: This study will assess the efficacy of the drug Camostat, currently approved in Japan for the treatment of pancreatitis and oesophagitis, two diseases characterised by inflammation. When used in vitro the drug was able to block the entry of SARS-CoV-2 into host cells. This study will assess the drug’s efficacy in COVID-19 positive patients in the community. It is hypothesised that treatment with Camostat will reduce disease progression and lead to a reduction in COVID-19-associated hospital admissions.
More details can be found on the SPIKE-1 study website:
Other Clinical Programmes
In addition to our STOPCOVID activities, on-going clinical programmes include:
1. LungSpy: We have developed novel platforms for distal pulmonary imaging, delivery and sampling. The optical imaging platform utilises chemical SmartProbes that are delivered to a patient’s distal lung via our fibre-based medical device ‘Panoptes’. In the presence of pathogens/inflammatory pathways, the SmartProbes fluoresce, and this signal is detected and imaged by Panoptes in conjunction with bespoke imaging systems. Differences in the inherent properties of cancerous tissue (relative to healthy tissue) also enables the imaging platforms to identify cancer tissue even without the use of additional SmartProbes.
This clinical study, scheduled to commence in Q3/4 2021, will assess the safety of novel lung imaging platforms in a small (~80) number of patients, and perform a preliminary assessment of the platform’s ability to visualise bacterial infection and neutrophil activity and differentiate between cancerous and non-cancerous tissue. The technology development along the translational pathway is supported by CARB-X and will be followed by the larger BAC2BAC study.
2. Wellcome BAC2BAC: Annually, around 20 million Intensive Care Unit patients worldwide require mechanical ventilation. Suspected pneumonia, with diagnostic uncertainty leads to over-prescription of antibiotics, and contributes to the development of antimicrobial resistance. This multi-site (Edinburgh, Belfast, Manchester, UCL) clinical validation study will assess the safety and diagnostic performance of our lung-imaging platform in mechanically-ventilated patients with suspected or confirmed respiratory infections. This study is funded by a Wellcome Translation Awardand recruitment of patients is scheduled to commence in early 2022, once sufficient safety and efficacy data has been gathered through LungSpy.
3. SNAP-IT: Critically ill patients are susceptible to ARDS. ARDS can occur as a result of numerous different causes, confers a high risk of death and resists the majority of pharmacological therapies tested to date. Current methods for diagnosis and stratification of severity of ARDS in patients are insufficient, with activation of host neutrophils implicated in the development of ARDS. In this Phase II study, we hope to demonstrate that a bespoke chemical probe administered in very small doses directly into the distal lung can rapidly and accurately detect activated neutrophils, and hence could be used to diagnose, monitor and stratify patients who are critically ill.
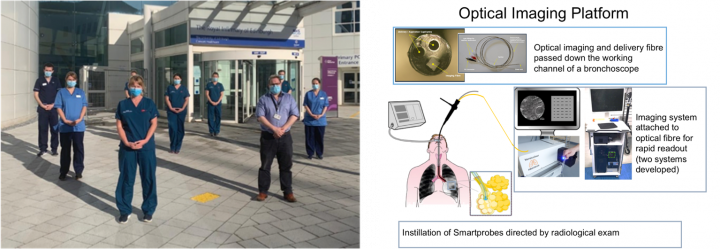
THT Clinical Research: (Left to Right) DEFINE study clinicial team; Lung imaging platform to be evaluated in LungSpy study.
Support and Facilities for Translation
The recently established Healthcare Technology Accelerator Facility (HTAF) in THT is crucially important for current and future commercialisation activities. HTAF unites Engineering and Physical Sciences groups from 5 universities with University of Edinburgh and NHS Lothian and is mentored by the California Life Sciences Institute and Codebase. Uniquely in a UK academic group, THT’s HTAF includes all the necessary expertise HTAF supports business development, legal and commercialisation expertise and aims to support spin offs, start-ups and industrial research. It also provides hands-on advice from expert partners to greatly accelerate and augment the translational/commercialisation process. HTAF is building links with international partners in India, USA, SMEs, and industry to organically grow an innovation ecosystem from the “bottom up”.
HTAF provides the following infrastructure to support technology development:
| Facilities | Capabilities |
| Physical science laboratories with associated biological validation facilities | Support for business development, commercialisation, product design, legal and trial design |
| Laboratories and clean rooms for instrumentation development and validation | Experienced Project and Clinical Project Managers |
| Manufacture and analysis of active pharmaceutical ingredients for early stage clinical studies | Direct portal for industry access/collaboration |
| MHRA accredited GMP Sterile liquid product manufacture | Embedded quality management systems (and associated personnel) for in-human technology development |
| Non-sterile manufacture, repackaging and blind labelling of Investigational Medicinal Products in a MHRA accredited unit | Regulatory specialists (medical devices and pharmaceuticals) |
| Translational multi-disciplinary scientists |

HTAF Translational Support: (Left to Right) Inaugural HTAF commercialisaion workshop; HTAF physical sciences workshop.
More details can be found on the HTAF website:
Selected THT Group Publications
- Mitros Z, Thamo B, Bergeles C, Da Cruz L, Dhaliwal K, Khadem M : Design and Modelling of a Continuum Robot for Distal Lung Sampling in Mechanically Ventilated Patients in Critical Care. Frontiers in Robotics and AI, 03 May 2021
- Craven TH, Walton T, Akram AR, Scholefield E, McDonald N, Marshall AD, Humphries DC, Mills B, Campbell TA, Bruce A: Activated neutrophil fluorescent imaging technique for human lungs. Scientific reports 2021; 11:1-15.
- Yerolatsitis S, Kufcsák A, Ehrlich K, Wood HA, Fernandes S, Quinn T, Young V, Young I, Hamilton K, Akram AR: Sub millimetre flexible fibre probe for background and fluorescence free Raman spectroscopy. arXiv preprint arXiv:201208836 2020.
- Wang Q, Hopgood JR, Finlayson N, Williams GO, Fernandes S, Williams E, Akram A, Dhaliwal K, Vallejo M: Deep Learning in ex-vivo Lung Cancer Discrimination using Fluorescence Lifetime Endomicroscopic Images: 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), IEEE, 2020, pp 1891-1894.
- Mills B, Megia-Fernandez A, Norberg D, Duncan S, Marshall A, Akram AR, Quinn T, Young I, Bruce AM, Scholefield E: Molecular detection of Gram-positive bacteria in the human lung through an optical fiber–based endoscope. European Journal of Nuclear Medicine and Molecular Imaging 2020:1-8.
- Fernandes S, Williams G, Williams E, Ehrlich K, Stone J, Finlayson N, Bradley M, Thomson RR, Akram AR, Dhaliwal K: Solitary pulmonary nodule imaging approaches and the role of optical fibre-based technologies. European Respiratory Journal 2020.
- Akram AR, Avlonitis N, Scholefield E, Vendrell M, McDonald N, Aslam T, Craven TH, Gray C, Collie DS, Fisher AJ: Enhanced avidity from a multivalent fluorescent antimicrobial peptide enables pathogen detection in a human lung model. Scientific reports 2019; 9:1-10.
- Krstajić N, Mills B, Murray I, Marshall A, Norberg D, Craven TH, Emanuel P, Choudhary TR, Williams GO, Scholefield E: Low-cost high sensitivity pulsed endomicroscopy to visualize tricolor optical signatures. Journal of biomedical optics 2018; 23:076005.
- Knighton N, Cottle B, Dentan V, Vercauteren T, Akram A, Bruce A, Dhaliwal K, Hitchcock R: Development of an Alveolar Transbronchial Catheter for Concurrent Fiber Optics-Based Imaging and Fluid Delivery. Journal of Medical Devices 2018; 12
- Craven TH, Avlonitis N, McDonald N, Walton T, Scholefield E, Akram AR, Walsh TS, Haslett C, Bradley M, Dhaliwal K: Super-silent FRET sensor enables live cell imaging and flow cytometric stratification of intracellular serine protease activity in neutrophils. Scientific reports 2018; 8:1-10.
- Akram AR, Chankeshwara SV, Scholefield E, Aslam T, McDonald N, Megia-Fernandez A, Marshall A, Mills B, Avlonitis N, Craven TH: In situ identification of Gram-negative bacteria in human lungs using a topical fluorescent peptide targeting lipid A. Science Translational Medicine 2018;10:eaal0033.
- Seth S, Akram AR, McCool P, Westerfeld J, Wilson D, McLaughlin S, Dhaliwal K, Williams CK: Assessing the utility of autofluorescence-based pulmonary optical endomicroscopy to predict the malignant potential of solitary pulmonary nodules in humans. Scientific reports 2016; 6:1-10.
- Krstajić N, Akram AR, Choudhary TR, McDonald N, Tanner MG, Pedretti E, Dalgarno PA, Scholefield E, Girkin JM, Moore A: Two-color widefield fluorescence microendoscopy enables multiplexed molecular imaging in the alveolar space of human lung tissue. Journal of biomedical optics 2016; 21:046009.
- Aslam T, Miele A, Chankeshwara SV, Megia-Fernandez A, Michels C, Akram AR, McDonald N, Hirani N, Haslett C, Bradley M: Optical molecular imaging of lysyl oxidase activity–detection of active fibrogenesis in human lung tissue. Chemical Science 2015; 6:4946-4953.
- Akram AR, Avlonitis N, Lilienkampf A, Perez-Lopez AM, McDonald N, Chankeshwara SV, Scholefield E, Haslett C, Bradley M, Dhaliwal K: A labelled-ubiquicidin antimicrobial peptide for immediate in situ optical detection of live bacteria in human alveolar lung tissue. Chemical Science 2015; 6:6971-6979.
- Mills, B., Akram, A., Norberg, D., Dhaliwal, K., Bradley, M., Megia-Fernandez, A. 2020. MMP-mediated Lung Tumour Painting. Chemical Communications. DOI: 10.1039/D0CC03886E.
- Gunasekaran, R., Lalitha. P., Williams. R., Bradley. M., Dhaliwal. K., Prajna. V., & Mills. B. 2020. Exploratory use of fluorescent SmartProbes for the rapid detection of microbial isolates causing corneal ulcer. American Journal of Ophthalmology. DOI: 10.1016/j.ajo.2020.06.014.
- Long, J., Parker, H., Ehrlich, K., Tanner, M., Dhaliwal, K., & Mills B. 2020. Frugal filtering optical lenses for point-of-care diagnostics. Biomedical Optics Express. DOI: 10.1364/BOE.381014.
- Ucuncu, M*, Mills, B*,. Duncan, S., Staderini, M., Dhaliwal, K., & Bradley, M. 2020. Novel Lipid A Binding Photosensitizer Probe for the Potent and Selective Killing of Gram-Negative Bacteria. Chemical Communications. DOI: 10.1039/D0CC00155D.

