Katrin Ottersbach: Developmental Origins of Blood Stem Cells and Leukaemia
Cancer Research Programme
Our group has a long-standing interest in how the first blood cells, particularly the first blood stem cells, are generated during development. We have identified a number of regulators that are involved in blood stem cell production, such as the transcription factor Gata3, the cell cycle regulator p57Kip2 and the signalling molecule Jak2, and have observed that mutations in these regulators have different effects on embryonic/foetal cells as compared with cells from the adult organism. This has revealed unique properties of embryonic/foetal blood cells with respect to the control of their cell cycle, self-renewal and response to DNA damage, and has obvious implications as to how these cells respond to disease mutations.
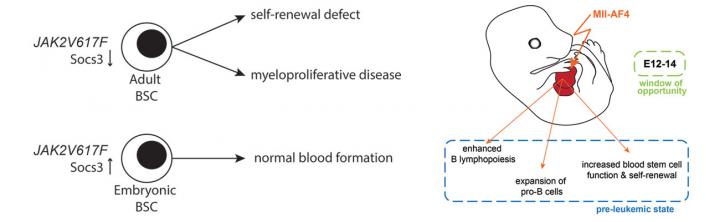
Indeed, we have recently uncovered that the JAK2V617F mutation, which is associated with myeloproliferative neoplasms in patients, in some cases progressing to acute myeloid leukaemia, and which was also been shown to have a negative impact on adult blood stem cells partly through increased DNA damage, has no impact on embryonic blood stem cell function (Mascarenhas et al. 2016). Specifically, embryonic blood stem cells are resistant to JAK2V617F-induced DNA damage, their repopulation and self-renewal capacity is maintained even in serial transplantations and they do not initiate myeloproliferative disease (Fig.1).
More recently, we have turned our attention to infant leukaemia characterised by the t(4;11) chromosomal translocation, which produces the MLL-AF4 oncogenic fusion. This disease has a known prenatal origin and a dismal prognosis. We targeted expression of MLL-AF4 to the earliest definitive blood cells that emerge in the embryo and found that this produced an increased B lymphoid output, with an expansion at the pro-B cell stage, which corresponds to the stage at which differentiation arrests in leukaemic patients (Barrett, Malouf et al. 2016). MLL-AF4 also conferred enhanced self-renewal potential. Furthermore, the effect was strongest in a particular blood progenitor during a restricted developmental window, thus identifying a window of opportunity and a potential cell-of-origin, in which this disease is likely to initiate (Fig.2). Future research efforts will focus on the molecular details of this pre-leukaemic state and a possible involvement of the foetal haematopoietic microenvironment, with the ultimate aim of establishing a faithful model of this disease in which drug treatments can be tested.