Margaret Frame
Research Programme
In recent years, we have shown that the FERM domain of FAK interacts with key regulators of directional migration and cancer cell polarity, namely Arp3 and the molecular scafold RACK1 (Serrels et al., 2007, Nature Cell Biology; Serrels et al., 2010, Current Biology). Since we and others find that FERM interactors control processes associated with cancer cell survival, invasion and metastasis, we are performing proteomics-based screens for FAK FERM domain-interacting proteins. There are multiple partners, and pathways, which are controlled via FERM interactions, including proteins that are involved in processes other than cell adhesion and migration. We are combining proteomics and pathway analysis to understand the FAK FERM interactome, and how key regulators of actin/adhesion networks at the cell periphery cause oncogenic perturbations in cell behaviour. We recently reviewed the potential wider roles of FERM interactions in cancer biology (our recent review; Frame et al., 2010, The FERM domain: organising the structure and function of FAK. Nature Reviews Molecular and Cellular Biology; image therein).
We have shown that tumour progression requires FAK in multiple epithelial cancer types, including skin (McLean et al., 2004, Genes and Development), breast and colon cancer – the latter two in genetically engineered mouse models. These were collaborations with the labs of Bill Muller (Montreal) and Owen Sansom (Glasgow), respectively (Lahou et al., 2007, PNAS; Ashton et al., 2010, Developmental Cell).
We have also demonstrated that Src inhibition suppresses metastasis in a genetically engineered mouse model of pancreatic cancer; specifically, dasatinib inhibits metastasis while having no effect on primary tumour cell proliferation (Morton et al., 2010, Gastroenterology). This is in keeping with our in vitro data that inhibitors of Src and FAK are likely to affect processes linked to cancer cell polarity, migration, invasion and metastasis. This is also backed up by recent in vivo data using optical windows and quantitative monitoring of invasive migration in the complex 3-dimensional tumour environment (Canel et al., 2010, Cancer Research).
We have been able to generate extremely useful cells from cancer models that are FAK deficient, and which can be reconstituted with wild-type FAK or signalling mutants. These have allowed us to determine the role of signalling through FAK, from integrins and from the upstream Src kinases, and via FAK’s kinase activity, in maintaining aspects of the cancer phenotype. These have led to several findings on control of E-cadherin dynamics in vitro and in vivo, and on proliferation in 3-dimensional environments (Canel et al., 2010, Cancer Research; Serrels et al, 2011, Molecular Cancer Therapeutics).
Perhaps the most striking new finding from our genetic deletion studies (in both cells and animal tissues), has been that integrin signalling through the Src/FAK axis regulates autophagy in advanced cancer cells. Importantly, as well as regulating the flux through the general autophagy pathway, FAK deletion causes severe loss of homeostasis and selective autophagic targeting of active Src and Ret. In turn, this leads to sequestration away from adhesion sites, and rapid autophagy-dependent lysosomal degradation of active Src, which cancer cells can use to survive (Sandilands et al., 2011, Nature Cell Biology; Sandilands et al., 2012, EMBO Reports).
An important aim of the translational part of our work is to monitor drug effects, and molecularly targeted interventions, by direct quantitative intra-vital imaging. This permits us to address: a) how pharmaceutical agents, and combinations, affect cancer development and tumour-associated phenotypes, particularly with respect to anti-invasive and anti-metastatic properties, b) how specific gene deletions affect tumour cell behaviour, and c) how cell and protein (or protein complex) dynamic measurements and pathway analyses may be used to gain mechanistic insight and monitor biological responses in high definition in vivo.
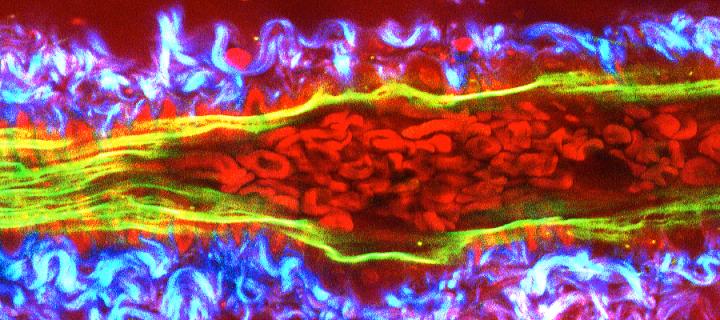
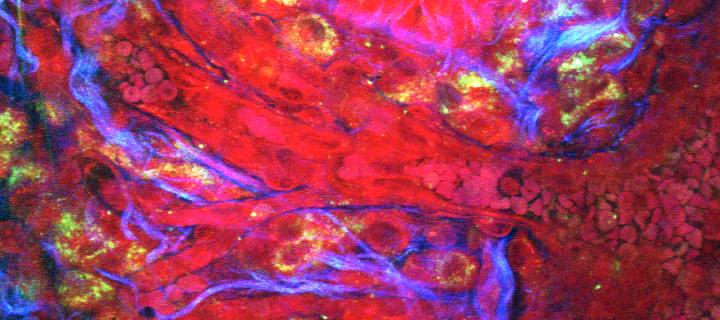
We are establishing protocols to generate fresh patient-derived cancer cells, and cells from genetically engineered models, which can be manipulated ex vivo to express specific new cancer-associated probes (which we are engineering; for example see Welman et al., 2010, J Biol Chem). These are being used to perform cancer cell behavior analysis in the complex 3-dimensional in vivo environment. Optical observation windows have been developed to permit direct and quantitative imaging of tumour/host interactions, ingression of host vasculature into tumour fragments (neo-angiogenesis), cancer cell movement and proliferative or survival responses deep into tissue in vivo.
Together with colleagues from the College of Science and Engineering in Edinburgh (Andy Downes and Alistair Elfick), we are developing label-free modes of tumour and drug imaging using forms of Raman Spectroscopy. Funding has been obtained from Cancer Research UK, the University of Edinburgh and the MRC Human Genetics Unit to develop a bespoke CARS/2-photon multi-modal imaging system in the Edinburgh Cancer Research Centre. (Images below courtesy of A. Serrels and M. Lee.)

