Yi Feng: Inflammation and Cancer
Cancer Research Programme
Background
Cancer of epithelial origin is initiated by a single cell transformation event and subsequent over-growth within an otherwise normal epithelial sheet environment. During this earliest cancer development stage, intimate interactions between the transformed-cell and its microenvironment are critical and will lead either to exclusion or nurturing so that any retained progeny may proceed onto full blown cancer.
Although the initial interaction between a preneoplastic cell (PNC) and its neighbouring host tissue is likely to be a major determinant of whether the PNC establishes a clonal niche and tumour progression ensues, this critical time period has proven intractable to experimental studies. There are few model systems in which the PNC clone can be visualised,in vivo, at its inception and its proliferative progression monitored as it interacts with host tissue. The amenability of zebrafish to genetic manipulation and the facility afforded for direct visualization of cellular events in situ, by live imaging, has created an opportunity to establish a novel model in which PNC:host interaction can be observed and manipulated in real time at the cellular/sub-cellular level.
It is acknowledged for more than a century that inflammation is linked to tumour progression. Emerging evidence suggests that signals derived from abnormal growth of PNC establish a tumour promoting inflammatory microenvironment. However, most studies are limited to analysis of later down-line aspects of cancer inflammation, whereas early and critical determinants of how such an inflammatory microenvironment is established, particularly the temporal aspects have proven difficult to study. But such knowledge may provide vital insights for the design of preventive approaches to malignant disease.
Recently we established a transgenic zebrafish larval model in which the oncogene HRASG12V fused with eGFP induces somatic cell transformation, and innate immune cells are visualised by expression of the complementary DsRed marker (Figure 1).

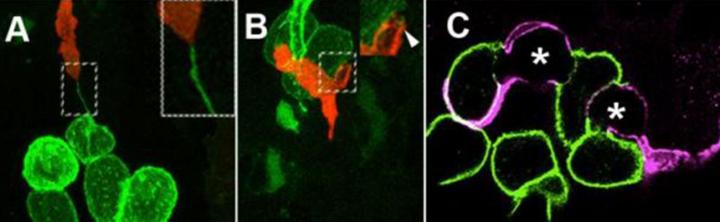
This combination enabled us for the first time, to visualize, the earliest interactions between PNC and leukocytes in vivo. Our data showed that PNCs induced an inflammatory response, by activating innate immune cells. We see recruitment of innate immune cells by 48hpf when transformed cells are still only singletons or doublets.
Soon thereafter we see intimate associations between immune and PNCs with frequent examples of cytoplasmic tethers linking the two cell types (Figure 3A, movie) as well as engulfment of PNCs by both neutrophils (Figure 3B) and macrophages (Figure 3C).
We show that a major component of the signal drawing inflammatory cells to oncogenic HRASG12V PNC is H2O2 which is also a key “damage cue” responsible for recruiting neutrophils to a wound. Strikingly, we found that recruited leukocytes produce Prostaglandin E2, which is one of the trophic factors that promote PNC growth. More importantly, we showed this trophic function of innate immunity, during the earliest stage of tumourigenesis, is suppressed by COX inhibitors, which provides one mechanistic explanation, as to why long term usage of Non-Steroidal Anti-inflammatory Drugs (e.g. Aspirin) is cancer preventive.
More recently we have developed an inducible tumourigenesis model in zebrafish, which allowed us to induce PNC in larval skin with a precise temporal and spatial control. Using this model together with signalling reporter fish that we have generated in the lab, we have begun to live image signalling events immediately after oncogene activation that are involved in regulating the Trophic inflammatory response. We also use the RNS-seq approach to identify gene expression changes in both PNCs and recruited innate immune cells.

