Alan Serrels: Tumour Immune Environment
Cancer Research Programme
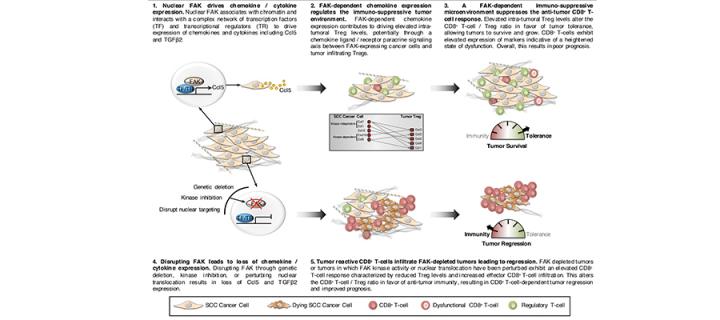
Reprogramming the tumour immune environment to promote an anti-tumour immune response has shown promise as a therapeutic approach in the fight against cancer. In preclinical murine models, targeting a variety of immune cell populations including macrophages and Tregs has shown anti-tumour efficacy either alone or when used in combination with agents that enhance CD8 T-cell activation. Our lab is interested in understanding how aberrantly regulated signalling pathways in cancer cells contribute to orchestrating the immuno-suppressive tumour environment, and how targeting these can be used to potentiate anti-tumour immunity either alone or in combination with other immunotherapies. In this context we have identified a new role for Focal Adhesion Kinase (FAK), a non-receptor protein tyrosine kinase frequently upregulated in cancer. Best known for its functions downstream of integrins and growth factors receptors, FAK can also translocate to the nucleus where its function remains poorly characterised.
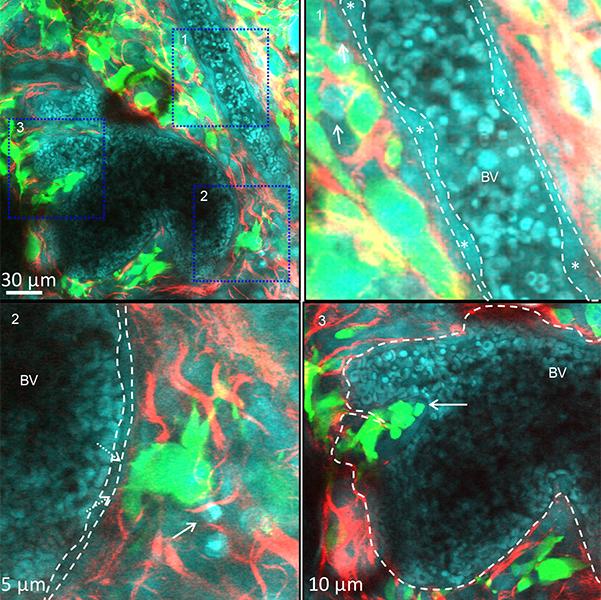
We have recently identified that nuclear FAK can regulate the transcription of chemokines and cytokines important in controlling the anti-tumour immune response (Figure 1) (Serrels et al, Cell, 163, Sept 2015). Furthermore, comparison of nuclear FAK levels between Squamous Cell Carcinoma (SCC) cells and skin keratinocytes (the normal cell counterpart to the SCCs) has shown elevated levels of nuclear FAK in cancer cells, implying that oncogenic transformation may trigger its nuclear accumulation. Thus, FAK represents a new target for immunotherapy, and modulating its nuclear function may yield cancer specific responses. Understanding the extent and mechanistic basis of FAK-dependent immune modulation in cancer, and identifying optimal combinations with other immunotherapies are key aims of the lab. To address our research questions, we use a combination of cutting edge genome editing, gene expression analysis, proteomics, flow cytometry, and advanced intra-vital imaging (Figure 2).

