Synergistic anticancer inhibitor combination discovered by a novel phenotypic screen
Scientists from the Cancer Research UK Edinburgh Centre used a novel chemical-genetic high-content phenotypic screen to discover synergistic anticancer effects of FAK and HDAC inhibitor combination: January 2020
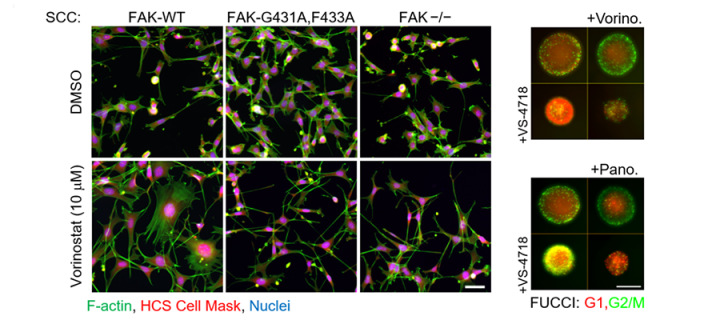
Focal adhesion kinase (FAK) is a protein that belongs to the family of so called non-receptor tyrosine kinases. It is frequently overexpressed in cancer, where it functions downstream of integrins (proteins mediating cell binding to the extracellular matrix) and growth factor receptors to regulate a diverse range of cellular functions, including survival, proliferation, adhesion, migration, angiogenesis and others. FAK therefore, plays a pivotal role in transducing signals from the plasma membrane and the nucleus and a number of FAK inhibitors are currently undergoing clinical development. However, it is evident from clinical studies to date that FAK inhibitors have limited effects on cancer cell growth and survival when used as monotherapy. In contrast, FAK’s role as a key signalling mediator of survival under conditions of cellular stress, including upon treatment of tumours with chemotherapy, suggests that targeting FAK in combination with other agents (that induce stress) may represent a more effective strategy.
Researchers from the CRUK Edinburgh Centre, University College Dublin and the Spanish National Cancer Research Centre developed an approach to identify new molecules that could form clinically actionable, synergistic combinations with FAK inhibitors. In a study led by Professors Margaret Frame and Neil Carragher and published recently in Molecular Cancer Therapeutics, a journal of the American Association for Cancer Research, they used cancer cells that were genetically engineered to express a mutated form of FAK which resembled pharmacological inhibition. This allowed them to perform a chemical-genetic combination screen to discover novel signalling pathways dependent upon FAK activity. The authors identified how inhibition of FAK affected the cellular phenotypes induced by a library of chemical inhibitors of other proteins in two-dimensional (2D) and three-dimensional (3D) tissue cultures - a strategy known as phenotypic screening. This approach led to identification of synergistic combinations between FAK inhibition and a number of other inhibitors, most notably of the histone deacetylase (HDAC) family of enzymes. The results were subsequently validated using combinations of clinically relevant FAK and HDAC inhibitors in a number of different cancer cell lines (including lung and esophageal cancer cells) and in xenograft models in vivo, confirming the initial findings. Mechanistically the combination of FAK and HDAC inhibitors is particularly effective in a subset of cancer cells in which FAK and HDAC inhibition blocks nuclear localization of a transcriptional regulator protein YAP (yes-associated protein 1), which is implicated in many types of cancer. It may be expected that these findings will invigorate further studies on the utility of FAK and HDAC inhibitors in the clinic.
Our work demonstrates power of chemical-genetic high-content phenotypic screens to find new synergistic anti-cancer combinations that may, in the future, be clinically actionable.
Related Links:
- Article in Molecular Cancer Therapeutics
- Professor Margaret Frame group website
- Professor Neil Carragher group website
- Edinburgh Cancer Discovery Unit web-page
- More information about phenotypic screening

