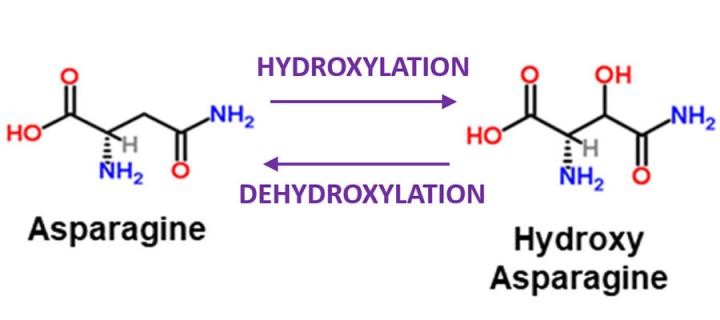
Asparagine hydroxylation is a reversible post-translational modification
A study led by Edinburgh researchers provided data indicating that asparagine hydroxylation is a flexible and dynamic post-translational modification: September 2020
Proteins are fundamental building blocks of every living organism. They consist of amino acids and perform multitude of functions – from acting as scaffolding and structural elements, to transporting molecules, driving DNA replication and catalysing metabolic reactions. Proteins are synthesized by ribosomes, which translate mRNA molecules into polypeptide chains. Each protein consists of one or more of such polypeptide chains.
Functions and life cycles of proteins are often regulated by so called posttranslational modifications - modifications occurring after their biosynthesis by ribosomes. A broad spectrum of such modifications have been described over the years. They include lipidation (attachment of lipids), glycosylation (attachment of carbohydrates), phosphorylation (attachment of a phosphoryl group) and others. Aberrations in posttranslational modifications of proteins are often observed in cancer and other diseases. It is therefore important to fully elucidate the nature and roles of various posttranslational modifications and their influence on localization and functions of different proteins.
Researchers from the Mass Spectrometry Facility in the Institute of Genetics & Molecular Medicine (IGMM) have particular interest in protein hydroxylation – introduction of hydroxyl group (-OH) into proteins’ amino acids. Amino acid hydroxylation is a common post-translational modification which generally regulates protein interactions or adds a functional group that can be further modified. Hydroxylation was believed to be irreversible, necessitating the degradation and re-synthesis of the entire protein to reset the modification. However, in a study titled “Asparagine hydroxylation is a reversible post-translational modification” which was recently published in the journal Molecular & Cellular Proteomics, IGMM scientists provided data indicating that asparagine hydroxylation is reversible. They postulated that protein hydroxylation is a flexible and dynamic post-translational modification akin to modifications involved in regulating signalling networks, such as phosphorylation, methylation and ubiquitylation. The study challenges previous believes and opens new frontiers in hydroxylation research. Better understanding of the process may provide key novel insights into intracellular signalling networks, possibly leading to discovery of new therapeutic targets to treat cancer or other human pathologies.
The work was driven by Dr Javier Rodriguez and led by Dr Alexander von Kriegsheim from the Cancer Research UK Edinburgh Centre. It was supported by Cancer Research UK, Wellcome Trust and the Victorian Cancer Agency Mid-Career Research Fellowship program.
Related Links:
Article in Molecular & Cellular Proteomics: https://www.mcponline.org/content/early/2020/08/05/mcp.RA120.002189.long
Doctor Alexander von Kriegsheim group website: https://www.ed.ac.uk/cancer-centre/research/von-kriegsheim-group
IGMM Mass Spectrometry facility webpage: https://www.ed.ac.uk/igmm/facilities/mass-spectrometry
IGMM Mass Spectrometry facility leaflet: https://www.ed.ac.uk/files/atoms/files/mass_spectrometry_laboratory_-_cruk_edinburgh_centre.pdf
An interesting animation on how proteins are produced in eukaryotic cells: https://www.youtube.com/watch?v=gG7uCskUOrA
An interesting presentation on protein modifications: https://www.youtube.com/watch?v=K2bGjTaNsnM

