New connection between ribosome biogenesis and cellular senescence
Cancer Research UK Edinburgh Centre researchers discover that inhibition of ribosome biogenesis protein LSG1 causes endoplasmic reticular disruption and cellular senescence: June 2019
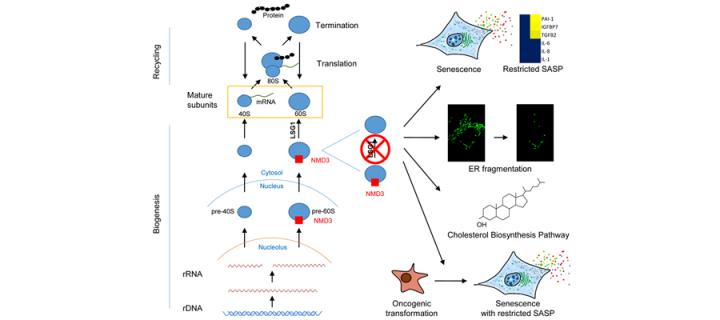
Cellular senescence is defined as irreversible cell cycle arrest that can be triggered by a variety of mechanisms. It is now appreciated to be much more than a passive cell-autonomous antiproliferative program reflecting the normal aging process. In fact, senescence is believed to be a key cellular program that can be induced and plays an important role in permanently restricting the propagation of damaged and defective cells. It is thought to be an important barrier to cancer initiation and there is considerable hope that senescence-linked molecular processes can be hijacked to develop novel anti-cancer and anti-aging therapeutics. Consequently, there is substantial ongoing research trying to elucidate molecular mechanisms implicated in regulation of cellular senescence, and the Cancer Research UK Edinburgh Centre/Institute of Genetics & Molecular Medicine scientists are at the forefront of developments in this area.
In a study titled “Inhibition of the 60S ribosome biogenesis GTPase LSG1 causes endoplasmic reticular disruption and cellular senescence” published recently in the journal “Aging Cell”, researchers from the Cancer Centre’s “Cell Metabolism and Homeostasis” and “Cellular Senescence and Tumour Suppression” groups, supported by Dr Tamir Chandra from the MRC Human Genetics Unit, provide important new insights into the links between cellular senescence and ribosome biogenesis. The project led by Drs Andrew Finch and Juan Carlos Acosta demonstrated that inhibition of the reaction catalysed by LSG1 leads to a robust induction of cellular senescence. LSG1 is involved in biogenesis of the 60S ribosomal subunit and ribosomes serve as sites of protein synthesis (translation), surprisingly however, the observed cellular senescence was associated with perturbation of endoplasmic reticulum (ER) homeostasis and upregulation of the cholesterol biosynthesis pathway rather than ribosome depletion or translational insufficiency. Furthermore, targeting of LSG1 resulted in amplification of the cholesterol/ER signature and restoration of a robust cellular senescence response in transformed cells, suggesting potential therapeutic uses of LSG1 inhibition.

