RAC1B is an important mediator of colorectal tumourigenesis
Cancer Research UK Edinburgh Centre scientists identify RAC1B as an important mediator of colorectal tumourigenesis and a potential target for enhancing the efficacy of EGFR inhibitor treatment: April 2021
Colorectal cancer (CRC), also known as bowel cancer, colon cancer, or rectal cancer, is the second commonest cause of cancer related mortality with highly variable disease outcomes and responses to therapies. It is more frequent in developed countries and risk factors include older age, high intake of fat, sugar, alcohol, red meat, processed meats, obesity, smoking, and a lack of physical exercise. More than 16,000 people die from CRC in the UK every year.
Colorectal cancers are very heterogeneous. Several subtypes with distinct features have been described to date and there is significant diversity between different subtypes at the molecular level. It is believed that better understanding of cellular events associated with CRC is fundamental for designing optimal treatments for individual patients and for reducing risks of therapy resistance.
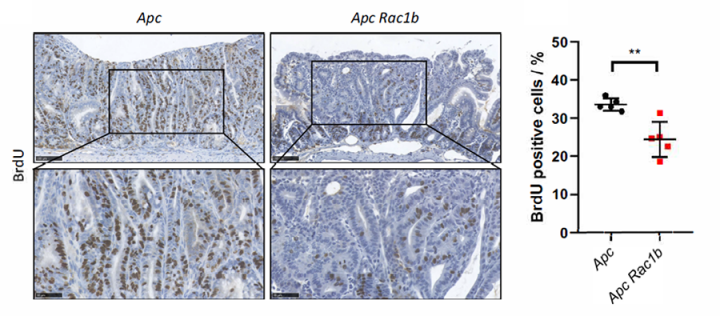
In a recent study, titled “RAC1B modulates intestinal tumourigenesis via modulation of WNT and EGFR signalling pathways”, researchers from the University of Edinburgh, CRUK Beatson Institute, Curtin University, University of Cambridge, Royal Holloway University of London and University of Glasgow, demonstrated that RAC1B - a splice variant of protein RAC1 - is an important mediator of colorectal tumourigenesis. Their research showed that high RAC1B expression in human colorectal cancer is associated with aggressive disease and poor prognosis. Additionally, deletion of RAC1B gene significantly increases survival and reduces tumour number, tumour cell proliferation and tumourigenic WNT signalling in a mouse colorectal cancer model. Importantly RAC1B interacts with a network of membrane bound receptor tyrosine kinases including members of the Epidermal Growth Factor Receptor (EGFR) family and is required for efficient activation of EGFR signalling. Notably, the study also demonstrated that RAC1B inhibition sensitised cetuximab (EGFR inhibitor) resistant human tumour organoids to the effects of EGFR inhibition suggesting that RAC1B may be a potential therapeutic target for improving the clinical efficacy of EGFR inhibitors in CRC.
The work, led by Dr Kevin Myant from the CRUK Edinburgh Centre, was published in the journal Nature Communications. It was supported by funding from Cancer Research UK, European Research Council and the Chief Scientist Office.
Our results provide new insights into the role of RAC1B in bowel cancer and suggest that this protein may represent a candidate therapeutic target for co-treatment with EGFR inhibitors. They may contribute to the development of novel therapeutic options that complement existing treatments and improve patient outcomes in the future.
Related Links:
Article in Nature Communications: https://www.nature.com/articles/s41467-021-22531-3
Doctor Kevin Myant Group website: https://www.ed.ac.uk/cancer-centre/research/myant-group
Information about bowel cancer: https://www.cancerresearchuk.org/about-cancer/bowel-cancer

