Identification of a signalling axis associated with immune suppression and poor prognosis in pancreatic cancer
Using animal modelling and human transcriptomic datasets, Edinburgh researchers demonstrated that FAK-IL6 signalling amplifies pathways associated with immune suppression and poor patient prognosis in pancreatic ductal adenocarcinoma.
There are around 10,500 new pancreatic cancer cases in the UK every year. Pancreatic ductal adenocarcinoma (PDAC) is the most prevalent type of pancreatic neoplasm and accounts for more than 90% of pancreatic cancer cases. Unfortunately, despite extensive clinical testing of potential therapies, major advances in the treatment of patients have not been forthcoming.
Inflammation has emerged to be a key mediator of pancreatic cancer development and progression. PDAC tumours are frequently infiltrated by a variety of immune cells, including a type of white blood cells known as T cells or T lymphocytes. T lymphocytes and other immune cells contribute to establishing of a tumour specific microenvironment (TME), which supports growth of cancer cells and tumour spread. The details of this process remain elusive, but it is known that behaviour of immune cells in TME can be influenced by chemokines and cytokines produced by cancer cells. Chemokines and cytokines are secreted proteins that are able to regulate and determine the nature of immune responses and control immune cell trafficking.
Edinburgh Cancer Research investigators at the Institute of Genetics and Cancer were amongst the first ones to discover an important role of a cell adhesion protein Focal Adhesion Kinase (FAK) in shaping the tumour microenvironment by regulating chemokine/cytokine and ligand-receptor networks and in promoting cancer immune evasion [Cell. 2015;163:160-73]. FAK is upregulated in several types of cancer and FAK inhibitors have been developed with hope that they might contribute to better cancer treatments in the future. These inhibitors are now being tested in clinical trials (for example as monotherapy or in combination with a form of immunotherapy known as immune checkpoint blockade), including trials for pancreatic cancer patients, but there is still a lot to be learnt before FAK inhibitors can be used as cancer therapeutics to their full potential.
As FAK is hyperactivated in human PDAC, researchers from the “Tumour Immune Environment” group led by Dr Alan Serrels undertook a project aiming to better understand role of FAK signalling in pancreatic cancer. They utilised a specially designed mouse model harbouring activating mutation in an oncogene k-ras and loss of function mutation in a tumour suppressor gene p53 (genetic changes frequently observed in human PDAC) in combination with cutting edge molecular biology techniques and analysis of publicly available human PDAC gene expression data (so called transcriptomics datasets). Some results from the project were published recently in the British Journal of Cancer in an article titled “FAK promotes stromal PD-L2 expression associated with poor survival in pancreatic cancer”.
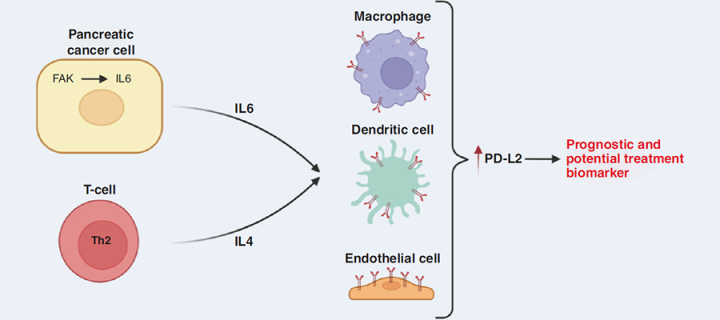
The authors demonstrated that FAK signalling in cancer cells stimulates them to secrete a cytokine interleukin-6 and that interleukin-6 can amplify actions of another cytokine, interleukin-4 (often produced by a type of T-lymphocytes known as CD4+ T-cells), in promoting expression of a Programmed Death Ligand 2 (PD-L2) protein on some cells in tumour microenvironment (e.g. macrophages, dendritic cells and endothelial cells). PD-L2 expression is known to contribute to tumour development by promoting immunosuppressive TME and the team has shown that PD-L2 expression in human PDAC is associated with tumour grade, clinical stage, molecular subtype and poor patient prognosis. Together, these results suggest that FAK inhibition could be beneficial in PDAC cases in which PD-L2 is upregulated.
The work, driven by Dr Catherine Davidson and supervised by Dr Alan Serrels, was supported by funding from Cancer Research UK and the Wellcome Trust.
Our data support the continued exploration of FAK as a potential therapeutic target for the treatment of pancreatic cancer. Future efforts should focus on understanding how to utilise FAK inhibitors and their immunomodulatory potential as part of rational drug combinations developed through a detailed understanding of FAK biology in PDAC and other cancers.
Related Links
Article in the British Journal of Cancer: https://www.nature.com/articles/s41416-022-01966-5
Dr Alan Serrels Group website: https://www.ed.ac.uk/cancer-centre/research/a-serrels-group
Information about pancreatic cancer: https://www.cancerresearchuk.org/about-cancer/pancreatic-cancer
“6 Warning Signs of Pancreatic Cancer” - interesting youtube video by the Cleveland Clinic: https://www.youtube.com/watch?v=S3CegUhEd7Y
Related Stories
Focal Adhesion Kinase controls transcription via chromatin accessibility
Focal Adhesion Kinase activation on lipid membranes
Kidney cancer drug accepted for use within NHS Scotland and across the UK
New strategy to target cancer cells expressing PD-L1
Licensing of a SRC/YES1 inhibitor with novel mode of action
The role of MYC in pancreatic adenocarcinoma
Synergistic anticancer inhibitor combination discovered by a novel phenotypic screen
Cancer immune evasion research on the cover of Science Signaling

